Why Is It Important That The Phospholipid Is Both Hydrophobic And Hydrophilic
4.6/5hydrophilichydrophobicanswer here
The lipid bilayer is arranged in two layers of phospholipids with the hydrophilic heads forming the outer edges and the tails forming the interior. This is important because it allows the bilayer to select which molecules it will allow into and out of the cell.
Furthermore, how does hydrophilic and hydrophobic relate to the cell membrane? Cell membranes are made up of a double layer of these phospholipid molecules. This is because in water the hydrophilic heads will face the water while the hydrophobic tails will be in the center because they face away from the water. The phospholipid bilayer makes the membrane very stable but also allows flexibility.
Keeping this in consideration, why are tails of phospholipids hydrophobic?
Phospholipids are amphipathic molecules. This means that they have a hydrophilic, polar phosphate head and two hydrophobic fatty acid tails. These components of the phospholipids cause them to orientate themselves, so the phosphate head can interact with water and the fatty acid tails cant, hence forming a bilayer.
What part of a phospholipid is hydrophilic?
A phospholipid consists of a head and a tail. The head of the molecule contains the phosphate group and is hydrophilic, meaning that it will dissolve in water. The tail of the molecule is made up of two fatty acids, which are hydrophobic and do not dissolve in water.
You May Like: Is Beef Bone Broth High In Cholesterol
B Lipids: Membranes And Fats
- Page ID
- 62484
A note from Dr. Haas: Lipids are molecules that are mostly nonpolar, but have some polar character. These molecules serve important biological functions, such as providing the principle component of membranes and serving as energy storage . The structures of a triglyceride and a phospholipid are shown above. Triglycerides are the things we commonly refer to as fats and oils. Phospholipids are similar to triglycerides with one important difference.
A skeletal structure of a phospholipid and a triglyceride are shown above. Notice the similarities and differences between the two structures. The phospholipid is similar to the triglyceride in that it contains fatty acid tails attached to a glycerol backbone. However, the phospholipid contains a organic phosphate zwiterion instead of a third fatty acid tail.
Triglycerides are completely insoluble in water. However, due to the ionic organic phosphate group, phospholipids demonstrate properties because the ionic group is attracted to water. Phospholipids have both a polar, hydrophilic end, and a nonpolar, hydrophobic end. Phospholipids are partially soluble in water, meaning that part of the molecule is attracted to water, and part of it is not. Phospholipids form important structures in water when the polar end faces water and the nonpolar end faces away from water. Below is a cartoon version of the phospholipid bilayer in 2D.
Understanding The Role Of Cholesterol In Cellular Biomechanics And Regulation Of Vesicular Trafficking: The Power Of Imaging
Issue title: 200th Anniversary of Cholesterol
Article type: Research Article
Affiliations: Departamento de Morfologia, Bloco J3, sala 310, Instituto de Ciências Biológicas, UFMG, Av. Antonio Carlos, 6627, 31270-901, Belo Horizonte, MG, Brazil. E-mail:
Keywords: Cholesterol, cell biomechanics, membrane trafficking, confocal microscopy, atomic force microscopy, laser tweezers, defocusing microscopy
DOI: 10.3233/BSI-160157
Journal: Biomedical Spectroscopy and Imaging, vol. 5, no. s1, pp. S101-S117, 2016
Abstract
Also Check: Are Pork Chops High In Cholesterol
Summary Lipophilic Vs Hydrophilic
The terms lipophilic and hydrophilic are adjectives which describe the solubility of compounds. The key difference between lipophilic and hydrophilic is that lipophilic refers to the ability of a substance to dissolve in lipids or fats while hydrophilic refers to the ability of a substance to dissolve in water or other hydrophilic solvents.
Reference:
1. Transport of Lipophilic Substances . com, Laboratory Continuing Education, Available here.
Image Courtesy:
2. 0302 Phospholipid Bilayer By OpenStax via Commons Wikimedia
Cholesterol Molecules Avoid Being Located In Adjacent Positions
We start by analyzing the pair correlation functions, g, for DPSC/Chol membranes at different Chol concentrations. The variable r stands for the radial distance from the center of mass of a cholesterol molecule and g measures density variation with respect to the average density as a function of distance. The results are shown in Figure 2. For a Chol-DSPC pair , g has its main peak at a distance of 0.5 nm. As this is also the first peak, it corresponds to the first coordination shell. In contrast, the main peak for Chol-Chol pair correlations is located at 1.0 nm. This is the second peak and hence corresponds to the second coordination shell. The physical interpretation of this observation is that cholesterols avoid being located in adjacent positions , . This behavior is common in all systems with moderate cholesterol concentrations. Only at high cholesterol concentrations does the occurrence of close contacts become relevant since then the membrane is crowded with cholesterol molecules.
Also Check: Shellfish High In Cholesterol
Can I Stop Taking Statins For A Week
Check with your doctor whether theres a particular time of day you should take your statin. You usually have to continue taking statins for life because if you stop taking them, your cholesterol will return to a high level within a few weeks. If you forget to take your dose, do not take an extra one to make up for it.
The Interaction Of Ibuprofen With Membranes Containing Cholesterol
| Fig. 7 Out-of-plane diffraction for bilayers prepared with 20 mol% cholesterol and ibuprofen concentrations of: 0 mol%, 5 mol%, 20 mol%. |
| Fig. 8 Electron density profiles for DMPC membranes prepared with 20 mol% cholesterol and 20 mol% cholesterol with 5 mol% ibuprofen . The curves in are on an absolute scale, while the ibuprofen containing curve in was scaled to overlap the profile with that of a 20 mol% cholesterol-containing DMPC membrane . The difference between the scaled curve and the black curve is best described by two Gaussian profiles, which are labelled in . |
Also Check: Does Beer Raise Cholesterol Levels
Cholesterol And Actin Cytoskeleton Organization: Imaging Cells Using Confocal Microscopy
Altering the levels of cholesterol in cellular membranes will interfere with rafts organization. Decrease in membrane cholesterol content, for example, leads to rafts disruption and consequently alters, directly or indirectly, the cellular processes linked to these regions, such as signaling, membrane trafficking and cytoskeleton organization. Cytoskeleton organization, in particular, seems to play an important role in rafts cellular functions. It has long been shown that membrane rafts are not only enriched in signal transduction molecules, but also actin and actin binding proteins . Additionally, it was demonstrated that changes in cytoskeleton organization upon rafts disruption also alters signaling processes linked to this platform .
Fig. 3.
Representative image of actin filaments and the sites of binding of phalloidin. Fluorescence images of mouse embryonic fibroblasts treated or not with MCD 10 mM, fixed with 4% paraphormadehyde and labeled with phalloiding conjugated with Alexa fluor 546 . Arrows indicate the actin stress fibers in MCD treated cells.
A lot of other work corroborated these data showing that cholesterol depletion from cell plasma membrane leads to actin polymerization and reorganization. Most importantly, many of these works showed that changes in the actin cytoskeleton induced cell stiffness and changes in biomechanical properties of cells .
The Study Of Cellular Biomechanics In Cholesterol Depleted Cells
As mentioned, the increase in actin stabilization at cell periphery and stress fiber formation leads to changes in cellular biomechanics. Cell actin organization, and consequently cell mechanics, is recognized to be a major player in various cell responses to internal and external environment , therefore the interest in studying the effects of plasma membrane cholesterol levels and rafts organization in cellular mechanics. A pioneer work in this field was published by Byfield and co-workers, working with aortic endothelial cells, where they showed that plasma membrane cholesterol content do relate with levels of membrane stiffness . After this, a lot of other papers were published. Most of them used microscopy techniques to study the biomechanical effects of cholesterol depletion induction of stress fiber formation. Below I will give a brief description of some of these techniques and the results obtained with them.
Recommended Reading: Does Black Coffee Affect Cholesterol
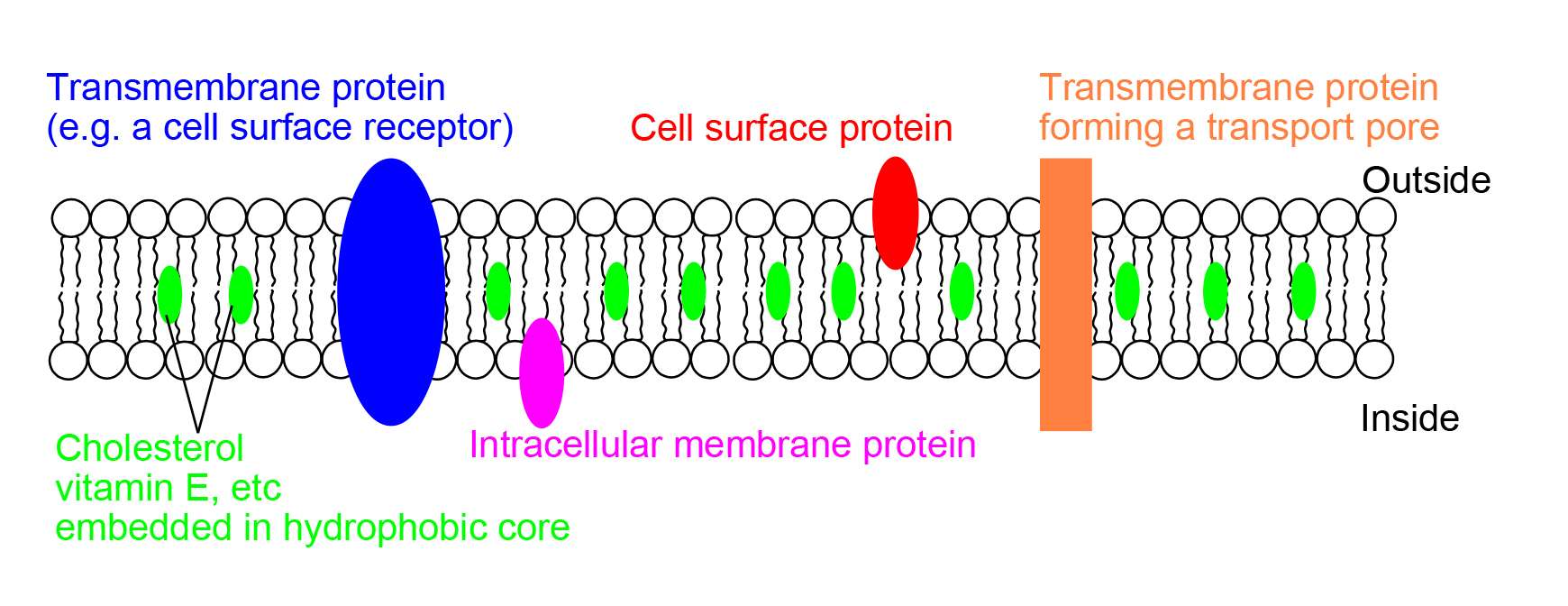
Role Of Cholesterol In Lipid Bilayer Conclusion
The lipid bilayer is a critical component in cellular membranes. This structure resembles a fluid mosaic and due to this characteristic, it is capable of controlling which substances get in and out of the cell, in addition to providing the cell with fluidity, another remarkable characteristic for the cells functionality.
Cholesterol is present in the lipid bilayer along with phospholipids and it plays a huge role in cell membrane capability.
Cholesterol inserts itself between phospholipids in the lipid bilayer and affects membrane fluidity not only by increasing the temperature range in which the cell membrane can continue to function, but it also acting as a barrier, as due to its chemical structure, it can fit in gap spaces, preventing water soluble substances from diffusing across the membrane.
Cholesterol Is Abundant In Cell Membranes
Cholesterol is found in every cell of your body. It is especially abundant in the membranes of these cells, where it helps maintain the integrity of these membranes, and plays a role in facilitating cell signaling meaning the ability of your cells to communicate with each other so you function as a human, rather than a pile of cells.
Molecule for molecule, cholesterol can make up nearly half of the cell membrane. Since it is smaller and weighs less than other molecules in the cell membrane, it makes up a lesser proportion of the cell membranes mass, usually roughly 20 percent.
Cholesterol is also present in membranes of organelles inside the cells, although it usually makes up a smaller proportion of the membrane. For example, the mitochondrion, the so-called power-house of the cell, contains only three percent cholesterol by mass, and the endoplasmic reticulum, which is involved in making and modifying proteins, is six percent cholesterol by mass.
Recommended Reading: Does Pasta Have High Cholesterol
Hydrophilic Vs Lipophilic Statins
Since the introduction of lovastatin in 1987 as the first 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor approved for human therapy, statins have become the most widely used lipid-lowering drugs with proven effect in cardiovascular disease prevention in different clinical settings . Statins have been classified in 3 categories based on their potency and efficacy in lowering low-density lipoprotein cholesterol concentrations. First-generation statins included lovastatin, pravastatin and fluvastatin, simvastatin and atorvastatin belong to the second generation, and rosuvastatin and pitavastatin to the third.
Chemical structure of hydrophilic and lipophilic statins.
Phospholipids And Biological Membranes
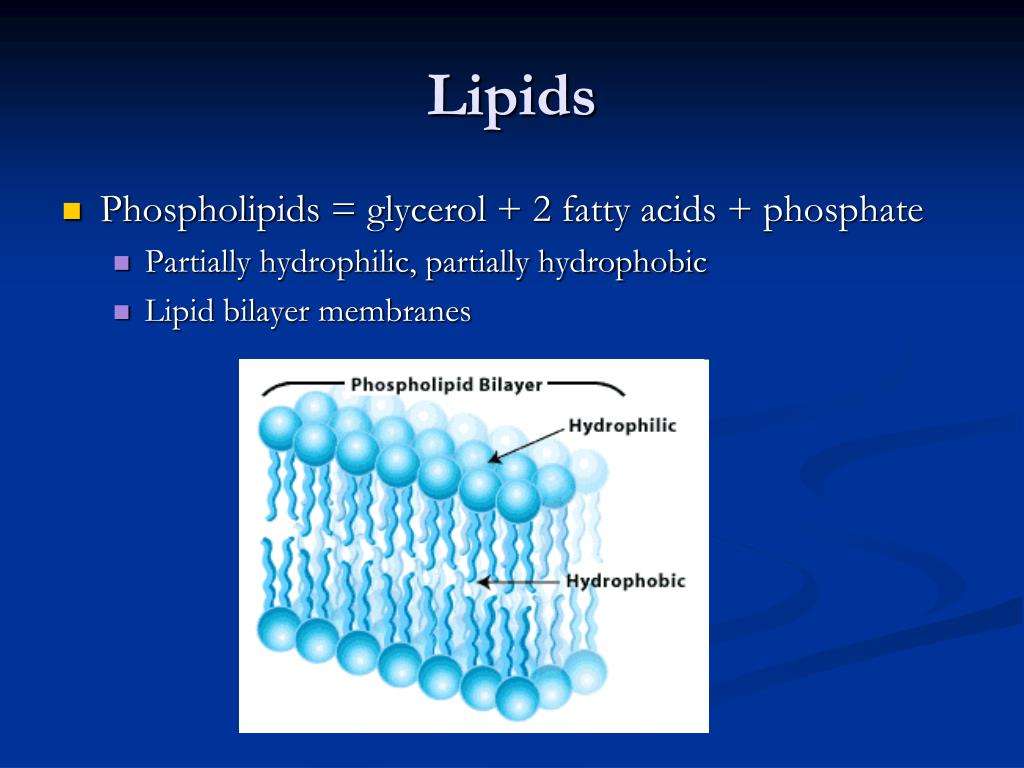
Figure 2. This illustration shows a phospholipid with two different fatty acids, one saturated and one unsaturated, bonded to the glycerol molecule. The unsaturated fatty acid has a slight kink in its structure due to the double bond.
Triglycerides are classified as simple lipids because they are formed from just two types of compounds: glycerol and fatty acids. In contrast, complex lipids contain at least one additional component, for example, a phosphate group or a carbohydrate moiety . Figure 2 depicts a typical phospholipid composed of two fatty acids linked to glycerol . The two fatty acid carbon chains may be both saturated, both unsaturated, or one of each. Instead of another fatty acid molecule , the third binding position on the glycerol molecule is occupied by a modified phosphate group.
Figure 3. Phospholipids tend to arrange themselves in aqueous solution forming liposomes, micelles, or lipid bilayer sheets.
Also Check: Does Feta Cheese Affect Cholesterol
Read Also: Do Ritz Crackers Have Fiber
Nuclear Magnetic Resonance Spectroscopy
31P-NMR spectroscopy is widely used for studies of phospholipid bilayers and biological membranes in native conditions. The analysis of 31P-NMR spectra of lipids could provide a wide range of information about lipid bilayer packing, phase transitions , lipid head group orientation/dynamics, and elastic properties of pure lipid bilayer and as a result of binding of proteins and other biomolecules.
Itioning Of Ibuprofen In Saturated Lipid Membranes With And Without Cholesterol
| Fig. 9 Measured difference in electron density with the addition of ibuprofen to DMPC membranes and calculated electron distributions of ibuprofen molecules. Three membrane bound states are fit to changes in electron density when 2 mol% ibuprofen is added to pure DMPC bilayers. The calculations take into account ibuprofen molecules, which extend into and are shared with neighbouring bilayers. Two embedded states are fit to the observed changes in electron density when 5 mol% ibuprofen is added to a membrane composed of DMPC + 20 mol% cholesterol. |
Three different membrane bound populations were observed when 2 mol% ibuprofen were added to the DMPC bilayers, as sketched in Fig. 9: a state in the hydrophobic membrane core, where the ibuprofen molecules align parallel to the lipid acyl chains 56% of ibuprofen molecules were found in this state 8% of ibuprofen molecules were observed at the interface between head groups-tail groups, and 36% of ibuprofen molecules were found attached to the membrane head group region, situated between the lipid head groups of two bilayers. At ibuprofen concentrations greater than 5 mol% , disruption of the lamellar membrane phase and the formation of a cubic lyotropic phase was observed.
Recommended Reading: Is Shrimp Bad For Your Cholesterol
Don’t Miss: Does Pasta Contain Cholesterol
Cholesterol Helps Maintain The Fluidity Of Cell Membranes
While cholesterol adds firmness and integrity to the plasma membrane and prevents it from becoming overly fluid, it also helps maintain its fluidity.
At the high concentrations it is found in our cells plasma membranes cholesterol helps separate the phospholipids so that the fatty acid chains cant come together and cyrstallize.
Therefore, cholesterol helps prevent extremes whether too fluid, or too firm in the consistency of the cell membrane.
Which Part Of A Lipid Is Polar
Lipids and Phospholipids Each lipid molecule contains a hydrophilic region, also called a polar head region, and a hydrophobic, or nonpolar tail region. Figure %: Basic Lipid Structure The hydrophilic region is attracted to aqueous water conditions while the hydrophobic region is repelled from such conditions.
Recommended Reading: Are Mussels High In Cholesterol
Don’t Miss: Is Mussels High In Cholesterol
Do Hydrophobic And Hydrophilic Attract
It is therefore erroneous to believe that only two hydrophobic entities attract each other when immersed in water: one hydrophobic and one hydrophilic entity usually also attract one another in water, albeit with a somewhat lower energy than is commonly seen with the attraction between two hydrophobic entities.
Read Also: Is Shrimp Bad For Your Cholesterol
Do Hydrophobic Or Hydrophilic Pass Through Membranes
The lipid bilayer is the main fabric of the membrane, and its structure creates a semipermeable membrane. The hydrophobic core impedes the diffusion of hydrophilic structures such as ions and polar molecules, but allows hydrophobic molecules, which can dissolve in the membrane, to cross it with ease.
Don’t Miss: Does Shrimp Raise Cholesterol Levels
Ion Pumps And Channels
Two special classes of protein deal with the ionic gradients found across cellular and sub-cellular membranes in nature- ion channels and ion pumps. Both pumps and channels are integral membrane proteins that pass through the bilayer, but their roles are quite different. Ion pumps are the proteins that build and maintain the chemical gradients by utilizing an external energy source to move ions against the concentration gradient to an area of higher chemical potential. The energy source can be ATP, as is the case for the Na+-K+ ATPase. Alternatively, the energy source can be another chemical gradient already in place, as in the Ca2+/Na+ antiporter. It is through the action of ion pumps that cells are able to regulate pH via the pumping of protons.
In contrast to ion pumps, ion channels do not build chemical gradients but rather dissipate them in order to perform work or send a signal. Probably the most familiar and best studied example is the voltage-gated Na+ channel, which allows conduction of an action potential along neurons. All ion pumps have some sort of trigger or âgatingâ mechanism. In the previous example it was electrical bias, but other channels can be activated by binding a molecular agonist or through a conformational change in another nearby protein.
Statin Solubility And Cardiovascular Outcomes
Statins inhibit cholesterol synthesis, thereby enhancing LDL clearance from the circulation. Since mevalonic acid is the precursor of numerous metabolites, HMG-CoA reductase inhibition potentially results in pleiotropic effects that may affect several tissue functions and modulate specific signal transduction pathways . These effects include anti-inflammatory and antioxidant activities, improvement in endothelial function, increased bioavailability of nitric oxide and delay in the progression of atherosclerotic plaques . All these vasculoprotective effects may account for a greater magnitude of and earlier time to cardiovascular benefit than apparently explained by changes in LDL cholesterol levels alone. However, leaving aside in vitro and experimental studies, it is not possible from available clinical evidence to isolate the potential benefits of pleiotropic effects of statins from those conferred by LDL cholesterol reduction.
Atheroprotective effects of statins. NO, nitric oxide LDL, low-density lipoprotein HDL, high-density lipoprotein.
One of the factors to be considered when evaluating outcomes of lipid-lowering treatment with statins is their solubility. Therefore, the role of both hydrophilic and lipophilic statins regarding beneficial pleiotropic effects and thus a possible improvement in cardiovascular primary or secondary prevention needs to be analysed further.
Also Check: Bone Broth Cholesterol
Role Of Cholesterol In Lipid Bilayer
The lipid bilayer is a thin biological membrane that is made of two lipid layers. Each layer is built with phospholipids that contain a hydrophilic head and a hydrophobic tail. This structure is fundamental for the functioning of a cellular membrane.
Cholesterol is one of the lipid components that are present in the lipid bilayer. This waxy substance belongs to the steroid family and it is crucial not only for the functionality of the cellular membrane, but also for human existence as it serves as a precursor to synthesize many of the bodys compounds, such as steroids hormones, vitamin D, and bile acids.
Keep on reading to learn about the cell membrane lipid bilayer structure, formation, components, the role of cholesterol in the lipid bilayer, and how this waxy substance affects membrane fluidity. Everything easily explained to facilitate full comprehension.
Lipid Bilayer Structure
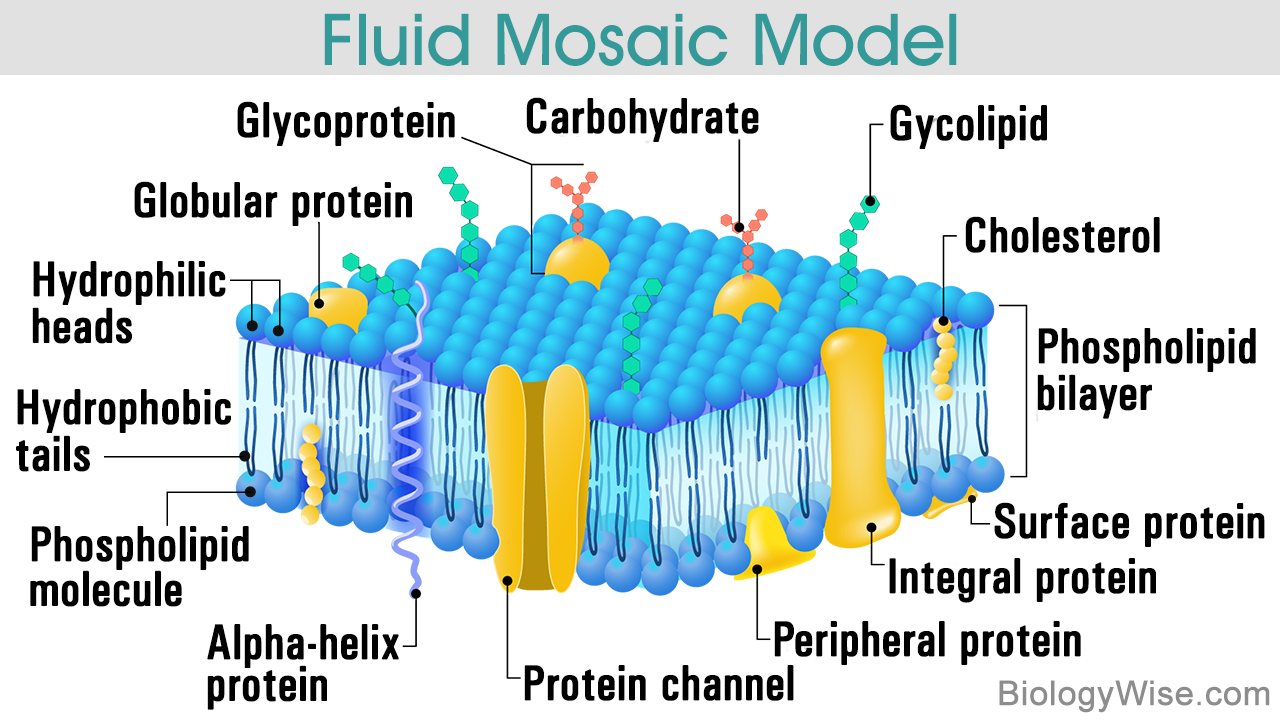
To start off, lets take a look at the lipid bilayer structure. Within the animals cell membrane, lipids constitute nearly 50% of its total mass. All of the lipids present in the cell membrane are amphipathic, which means that they possess a hydrophilic and a hydrophobic end.
The most predominant lipids are phospholipids, they have a hydrophilic head group and a hydrophobic tail, which are usually fatty acids.
The fatty acids tails can differ from each other in different aspects. One potential difference is their length, usually, they contain between 14 and 24 carbon atoms.
Fluid Mosaic Model