Phospholipids And Glycolipids Form Cellular Membranes
Perhaps the most important and basic function of lipids in living cells is in the formation of cellular membranes. All cells, from the most basic bacterium to those that form the most specialized human tissues, are surrounded by a plasma membrane made of lipid molecules. For more detail, see the Membranes I: Introduction to Biological Membranes module.
The lipids that form membranes are a special type called phospholipids . They are so named because they have a characteristic phosphate group . Like triglycerides, the central structure of a phospholipid is the glycerol molecule. However, phospholipids have two fatty acid tails attached to the glycerol, whereas triglycerides have three. On the remaining carbon of the glycerol, a large, charged, phosphate-containing group is added.
Figure 11Figure 12
Comprehension Checkpoint
Table 3110percentiles For Plasma Total High
- Increases in dietary cholesterol and fat raise levels of apoprotein E-containing lipoproteins in the plasma of man.Cole TG, Patsch W, Kuisk I, Gonen B, Schonfeld G. J Clin Endocrinol Metab. 1983 Jun 56:1108-15.
- Heterogeneous properties of intermediate- and low-density lipoprotein subpopulations.Srisawasdi P, Vanavanan S, Rochanawutanon M, Pornsuriyasak P, Tantrakul V, Kruthkul K, Kotani K. Clin Biochem. 2013 Oct 46:1509-15. Epub 2013 Jul 2.
- Review .Sandhofer F. Wien Med Wochenschr. 1994 144:286-90.
- .Bauchart D, Levieux D. Reprod Nutr Dev . 1985 25:243-50.
- Review .
The Lipid Bilayer Is A Two
It was only around 1970 that researchers first recognized that individual molecules are able to diffuse freely within lipid bilayers. The initial demonstration came from studies of synthetic lipid bilayers. Two types of preparations have been very useful in such studies: bilayers made in the form of spherical vesicles, called , which can vary in size from about 25 to 1 μm in diameter depending on how they are produced and planar bilayers, called , formed across a hole in a partition between two compartments .
A cross-sectional view of a black membrane, a synthetic lipid bilayer. This planar bilayer appears black when it forms across a small hole in a partition separating two aqueous compartments. Black membranes are used to measure the permeability properties
Phospholipid mobility. The types of movement possible for phospholipid molecules in a lipid bilayer.
Recommended Reading: What Raises Ldl Cholesterol Levels
Nomenclature Of Fatty Acids
For the application of the âÏâ nomenclature and considering the “order” of double bonds in unsaturated fatty acids having unconjugated stucture, it can be observed that by pointing the location of the first double bond, it will automatically determined the location of the subsequent double bonds . Thus, C18: 1 Ï-9, which has a single double bond at C9 counted from the methyl end, correspond to oleic acid , which is the main exponent of the Ï-9 family. Oleic acid is highly abundant both in vegetable and animal tissues. C18: 2 Ï-6 corresponds to a fatty acid having double bonds at the C6 and C9 . This is linoleic acid , the main exponent of the Ï-6 family and which is very abundant in vegetable oils and to a lesser extent in animal fats . C18: 3, Ï-3 corresponds to a fatty acid having double bonds at C3, C6 and C9. It is alpha-linolenic acid , the leading exponent of the Ï-3 family. ALA is a less abundant fatty acid, almost exclusively present in the vegetable kingdom and specifically in land-based plants . Within , C20: 4, Ï-6 or arachidonic acid C20: 5, Ï-3 or eicosapentaenoic acid and C22 : 6, Ï-3 or docosahexaenoic acid , are of great nutritional importance and are only found in ground animal tissues and in aquatic animal tissues and in plants of marine origin .
Figure 1.
Classification of fatty acids according to their degree of saturation and unsaturation and considering the notation ” Ï”.
| Nomenclature |
What Do Cholesterol And Phospholipids Do
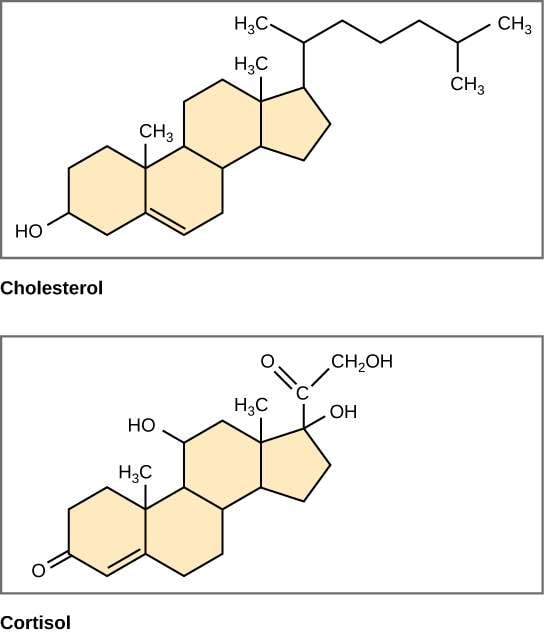
Cholesterol increases the fluidity of the hydrocarbon chains below and decreases the hydrophobicity of those above, by inducing an intermediate state in the phospholipid molecule with which it interacts. This is the basis for the formation of lipid droplets in the lipid bilayer.
Phosphatidylinositol-3-phosphate is an essential cofactor for lipoprotein lipase , the enzyme that catalyzes the conversion of triglycerides to triglyceride-rich particles and cholesterol to cholesterol. LPL is also involved in cholesterol biosynthesis. In the presence of high concentrations of free fattyacids , PI3K is phosphorylated, resulting in a decrease in its activity.
The phosphoinositide 3,4-P family of phosphatases, which catalyze the degradation of lipids, is activated by high levels of FFA and is responsible for lipid synthesis in adipose tissue. However, when the concentration of FFAs is too low, the activity of PIP 3 is decreased, leading to the accumulation of fatty acyl-CoA , which in turn leads to fatty liver disease .
Also Check: What Not To Eat On A Low Cholesterol Diet
Role Of Cholesterol In Lipid Bilayer
The lipid bilayer is a thin biological membrane that is made of two lipid layers. Each layer is built with phospholipids that contain a hydrophilic head and a hydrophobic tail. This structure is fundamental for the functioning of a cellular membrane.
Cholesterol is one of the lipid components that are present in the lipid bilayer. This waxy substance belongs to the steroid family and it is crucial not only for the functionality of the cellular membrane, but also for human existence as it serves as a precursor to synthesize many of the bodys compounds, such as steroids hormones, vitamin D, and bile acids.
Keep on reading to learn about the cell membrane lipid bilayer structure, formation, components, the role of cholesterol in the lipid bilayer, and how this waxy substance affects membrane fluidity. Everything easily explained to facilitate full comprehension.
Lipid Bilayer Structure
To start off, lets take a look at the lipid bilayer structure. Within the animals cell membrane, lipids constitute nearly 50% of its total mass. All of the lipids present in the cell membrane are amphipathic, which means that they possess a hydrophilic and a hydrophobic end.
The most predominant lipids are phospholipids, they have a hydrophilic head group and a hydrophobic tail, which are usually fatty acids.
The fatty acids tails can differ from each other in different aspects. One potential difference is their length, usually, they contain between 14 and 24 carbon atoms.
Fluid Mosaic Model
What Is The Key Difference Between Phospholipids And Fats
Phospholipids have glycerol, two fattyacids and phosphorus, which is not a fat. The formation of lipid bilayers is more dependent on Phospholipids than it is on Triglycerides. Fatcells store triglycerides and break them down. Fattyacids are the most abundant macronutrients in the human diet. They are found in animal products such as meat, fish, eggs, dairy products, nuts, seeds, vegetables, fruits, and cereals.
In contrast, carbohydrates are made up of glucose, fructose, sucrose, maltose, galactose and other monosaccharides. Carbohydrates can be broken down into glucose and fructose. Fructose is converted to glucose by the liver and stored as glycogen. Glucose is then used for energy, whereas fructose is used as a source of energy for the body. Glycogen is a type of stored carbohydrate that is stored in muscle and fatcells.
The body uses glucose as its primary energy source, but it can also use fructose as an energy-rich source. It is important to note that fructose and glucose are metabolized in different ways.
Also Check: What Foods Have Good Cholesterol
Which Is The Membrane Phospholipid
A phospholipid is a lipid that contains a phosphate group and is a major component of cell membranes. … In water, phospholipids spontaneously form a double layer called a lipid bilayer in which the hydrophobic tails of phospholipid molecules are sandwiched between two layers of hydrophilic heads .
Lipids Are A Source Of Energy
While both carbohydrates and lipids provide the fuel to energize your body, carbohydrates are the most readily available source of energy, and lipids function primarily as the bodys backup energy reserves, according to Virtual Chembook. However, your body is able to store far more energy in the form of lipids than in glycogen, the carbohydrate-based energy stores. When glycogen available to the body is exhausted, it begins to use lipid-based energy. Gram for gram, lipids outstrip glycogen in terms of available energy by more than two to one. A gram of lipids yields 9 kilocalories, compared with 4 kilocalories in every gram of glycogen. On the downside, particularly for those watching their weight, high-fat foods contain more than twice the calories per gram as foods that are high in carbohydrate.
Don’t Miss: Does Cholesterol Increase Membrane Fluidity
Do Phospholipids Have A Similar Chemical Structure To Cholesterol
Cholesterol is an important component in lipoproteins, which are the main components of the blood, and has some similar chemical properties to phospholipids. Protein is made up of aminoacids. Aminoacids can be broken down into smaller units called aminogroups. Each aminogroup has a specific function in the body.
For example, the amino acid leucine is a building block of protein, but it also plays a role as a cofactor in many enzymes, such as the enzyme that breaks down glucose into glucose-6-phosphate and glycogen . In addition, it is important for your body to be able to use protein as an energy source.
This is why protein is so important to your overall health and well-being.
What Are Lipid Rafts
Lipid rafts are possible areas of the cell membrane that contain high concentrations of cholesterol and glycosphingolipids. The existence of lipid rafts has not been conclusively established, though many researchers suspect such rafts do indeed exist and may play a role in membrane fluidity, cell-to-cell communication, and infection by viruses.
You May Like: Is Garlic Good For Lowering Cholesterol
Do Hydrophobic And Hydrophilic Attract
It is therefore erroneous to believe that only two hydrophobic entities attract each other when immersed in water: one hydrophobic and one hydrophilic entity usually also attract one another in water, albeit with a somewhat lower energy than is commonly seen with the attraction between two hydrophobic entities.
Read Also: Is Shrimp Bad For Your Cholesterol
Fats And Oils Store Energy
Animals and plants use fats and oils to store energy. As a general rule, fats come from animals and oils come from plants. Because of slight differences in structure, fats are solid at room temperature and oils are liquid at room temperature. However, both fats and oils are called triglycerides because they have three fatty acid chains attached to a glycerol molecule, as shown in Figure 3.
The carbon-hydrogen bonds found in the long tails of fatty acids are high-energy bonds. Thus, triglycerides make excellent storage forms of energy because they pack many high-energy C-H bonds into a compact structure of three tightly packed fatty acid tails. For this reason, dietary fats and oils are considered “calorie dense.” When animals, including humans, consume fats and oils, a relatively small volume can deliver a large number of calories. Animals, particularly carnivores, are drawn to high-fat foods for their high caloric content.
Triglycerides are formed inside plant and animal cells by attaching fatty acids to glycerol molecules, creating an ester linkage. This reaction is called a dehydration synthesis because a water molecule is formed by “pulling out” two hydrogen atoms and an oxygen from the reactants. Because a new water molecule is formed, this new reaction is also called a condensation reaction .
Figure 4
Comprehension Checkpoint
Fats that we eat are calorie-dense because
You May Like: How Much Cholesterol In Ice Cream
How Do Triglycerides Phospholipids And Sterols Differ In Composition And Structure
Almost all of the fat in the foods we eat is composed of triacylglycerols. emulsifiers bring water and fat together. Cells and carrier Molecules are made up of Phospholipids. Animals and plants have sulfates in their tissues. Sterols can be broken down by the body into fattyacids, cholesterol, and triglycerides. Cholesterol is the most common form of cholesterol in our bodies.
It is also called âgood cholesterolâ because it is necessary for the proper functioning of the heart and other organs. The body can convert cholesterol to other forms, such as HDL , which is associated with a lower risk of heart disease and stroke. However, HDL is not as effective as LDL, the âbad cholesterol,â which can lead to heart attacks and strokes.
Isomerism Of Fatty Acids
According to the distribution of double bonds in a fatty acid and to its spatial structure, unsaturated fatty acids may have two types of isomerism: geometrical isomerism and positional isomerism. By isomerism it is referred to the existence two or more molecules having the same structural elements , the same chemical formula and combined in equal proportions, but having a different position or spatial distribution of some atoms in the molecule .
Don’t Miss: Are Crabs High In Cholesterol
What Does The Bilayer Do
Cell membranes protect and organize cells. All cells have an outer plasma membrane that regulates not only what enters the cell, but also how much of any given substance comes in allowing only certain molecules to cross.
In addition to lipids, which account for approximately half the mass of the cell membrane, there are also peptides from DNA, cholesterol, and fatty acids to help our cells perform the integral metabolic functions of life. Cholesterol helps regulate the rigidity of membranes, while other less prominent lipids play roles in cell signaling and cell recognition.
The outer part of the cell membrane contains phosphatidylcholine and sphingomyelin . The inner contains phosphatidylethanolamine , phosphatidylinositol , and phosphatidylserine . The outer cell membrane leaflet predominantly contains PC and sphingomyelin while the inner membrane leaflet contains PE and PI.
What Are The Primary Functions Of Phospholipids
Phospholipids are prevalent in the cells of bacteria and eukaryotes. They are molecules made of a phosphate head and a lipid tail. The head is considered water-loving or hydrophilic, whereas the tail is hydrophobic, or repellent to water. Phospholipids are therefore called amphiphilic. Because of this dual nature of phospholipids, many types arrange themselves into two layers in a watery environment. This is called a phospholipid bilayer. Phospholipid synthesis occurs primarily in the endoplasmic reticulum. Other areas of biosynthesis include the Golgi apparatus and mitochondria. Phospholipids function in various ways inside cells.
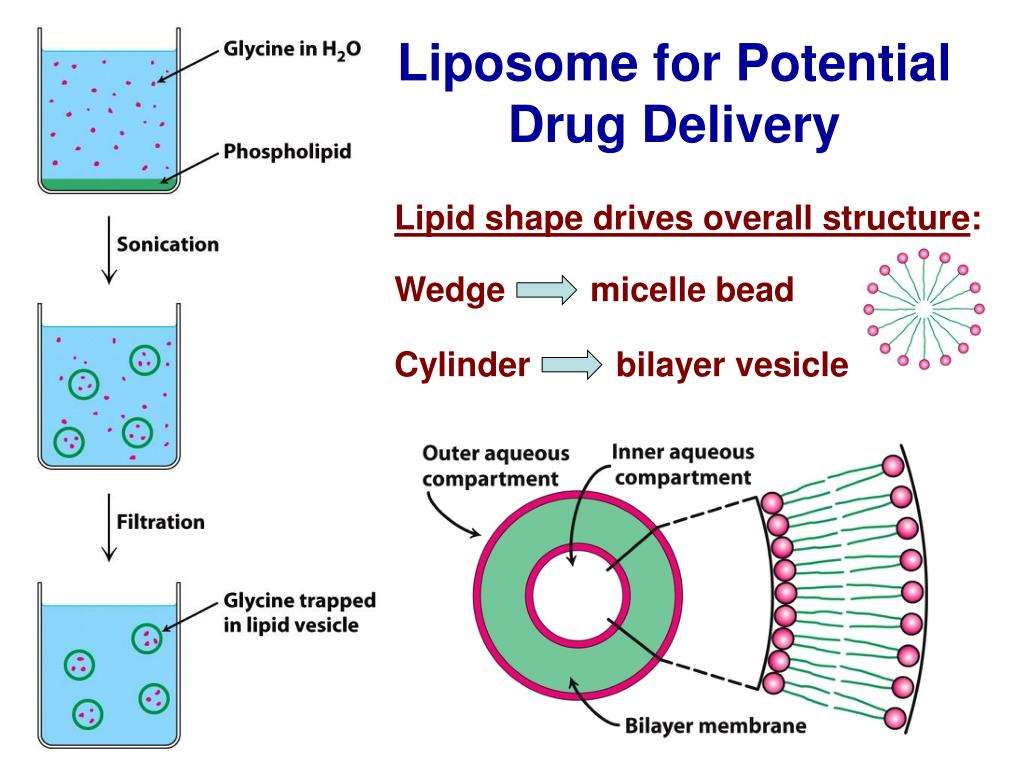
TL DR
Phospholipids are molecules with hydrophilic phosphate heads and hydrophobic lipid tails. They comprise cellular membranes, regulate certain cellular processes, and possess both stabilizing and dynamic qualities that can aid in drug delivery.
Don’t Miss: What To Do Before Cholesterol Test
What Are 3 Differences Between Phospholipids And Other Lipids
Phospholipids have a phosphate group in their molecule. There are examples of phospholipids. The class of organic compounds called lipids are insoluble in water. Waxes, natural oils, fattyacids, and fatty alcohols are examples of lipids.
The term âlipidâ is also used to refer to a group of molecules that have the same chemical structure as a lipid. For example, a lipoprotein is a type of lipid that is composed of a protein and a glycoprotein.
Lipids can be classified into three main groups: triglycerides , cholesterol , and non-starch polysaccharide .
Lipids: What Are Lipids Phospholipids And Cholesterol
Lipids are involved mainly in long-term energy storage. They are generally insoluble in polar substances such as water. Secondary functions of lipids include structural components and messengers that play roles in communications within and between cells. Lipids are composed of three fatty acids covalently bonded to 3-carbon glycerol. The fatty acids are composed of CH2 units and are hydrophobic/not water-soluble.
Fatty acids can be saturated or unsaturated . A fat is solid at room temperature, while oil is a liquid under the same conditions. The fatty acids in oils are mostly unsaturated, while those in fats are mostly saturated. Some examples of fatty acids are shown in Figure 1.
Figure 1
Diets are attempts to reduce the number of fats present in specialized cells known as adipose cells that accumulate in certain areas of the human body. By restricting the intake of carbohydrates and fats, the body is forced to draw on its stores to make up the energy debt. The body responds to this by lowering its metabolic rate, often resulting in a drop of energy level.
Successful diets usually involve three things: decreasing the amounts of carbohydrates and fats exercise and behavior modification. Fats and oils function in long-term energy storage.
Another use of fats is as insulators and cushions. The human body naturally accumulates some fats in the posterior area. Subdermal fat plays a role in insulation.
Also Check: How Accurate Are Finger Prick Cholesterol Tests
Phospholipid Intake In Humans
The normal dietary intake of PL is 28 grams per day, which represents 110% of total daily fat intake. Foods with a high PL content include eggs, organ and lean meats, fish, shellfish, cereal grains and oilseeds. Leafy vegetables, fruits and tubers, on the other hand, contain relatively low levels . The most common PL in food is phosphatidylcholine while other PLs, such as phosphatidylethanolamine , phosphatidylserine and phosphatidylinositol are present in much smaller amounts. Sphingomyelin , a phosphorus-containing lipid , is present in eggs, meat and fish and is ingested at a level of 0.30.4 grams per day. Production of low-fat food products has generally led to a reduction in dietary PL intake. It must not be overlooked however that PL , is widely used as a food additive. Lecithin is a standard ingredient in margarine, and provides consistency of texture to dressings and other creamy products. It acts as an important emulsifier in the manufacture of chocolate and aids in the dispersibility of food powders . Lecithin is also frequently used as a tin or mould release agent in the bakery and confectionery industry, and can form complexes with starch to improve crumb softness and shelf life of bread. Although levels of lecithin in processed foods are generally low , their widespread consumption implies that the amount of PL ingested in this form is not negligible.
The Interaction Of Ibuprofen With Membranes Containing Cholesterol
| Fig. 7 Out-of-plane diffraction for bilayers prepared with 20 mol% cholesterol and ibuprofen concentrations of: 0 mol%, 5 mol%, 20 mol%. |
| Fig. 8 Electron density profiles for DMPC membranes prepared with 20 mol% cholesterol and 20 mol% cholesterol with 5 mol% ibuprofen . The curves in are on an absolute scale, while the ibuprofen containing curve in was scaled to overlap the profile with that of a 20 mol% cholesterol-containing DMPC membrane . The difference between the scaled curve and the black curve is best described by two Gaussian profiles, which are labelled in . |
Recommended Reading: Is Bacon High In Cholesterol