How Do Triglycerides Phospholipids And Sterols Differ In Composition And Structure
There are three types of lipids in the body, triglycerides, phospholipids and sterols. Triglycerides are also known as triacylglycerols and compose 95% of fat in the foods we eat. … Phospholipids make up cell membranes and lipid carrier molecules. Sterols are found in tissues of animals and plants.
Can I Stop Taking Statins For A Week
Check with your doctor whether there’s a particular time of day you should take your statin. You usually have to continue taking statins for life because if you stop taking them, your cholesterol will return to a high level within a few weeks. If you forget to take your dose, do not take an extra one to make up for it.
Is Lipids Hydrophobic Or Hydrophilic
4.4/5LipidsLipidslipidlipidlipidhydrophilichydrophobic
fats, lipids). However, there are categories of lipids that are both hydrophobic and hydrophilic. Thus, lipids are largely insoluble in polar solvents , and are soluble in nonpolar molecules. As such, the nonpolar regions are hydrophobic/lipophilic, and the polar regions are hydrophilic/lipophobic.
Furthermore, are lipids generally hydrophilic or hydrophobic? Although lipids are amphiphatic molecules , lipids are generally hydrophobic due largely in part to their large proportion of hydrocarbons to polar regions .
In this manner, why are lipids hydrophilic?
The hydrophilic end interacts with the water molecules while the hydrophobic tail of the molecule retains its hydrophobic nature. They are also present in soaps where the combination of a hydrophobic tail and hydrophilic head allows other lipids to be dissolved into water.
Is cholesterol hydrophobic or hydrophilic?
Cholesterol is quite different in structure from the other membrane lipids that have been discussed. However, in common with most membrane lipids, cholesterol is an amphipathic molecule, containing both a hydrophobic portion and a hydrophilic portion, the hydroxyl. Figure 2.13.
Recommended Reading: Are Egg Beaters Low In Cholesterol
The Interaction Of Ibuprofen With Dmpc Membranes
| Fig. 4 Out-of-plane X-ray diffraction (q|| |
< 0.21 Å1 in more detail, as compared to the overview plots in Fig. 2. The pure DMPC bilayers in Fig. 6 show the lamellar L Bragg peak and two diffuse contributions: the lamellar diffuse scattering occurring in horizontal sheets is the result of bilayer undulation dynamics. Bilayers, which are not perfectly oriented parallel to the silicon substrate lead to a faint powder ring, labeled as defect scattering. The number of these defect bilayers is typically very small as evidenced by the logarithmic intensity plot.
| Fig. 6 High resolution reciprocal space maps show the increase in powder scattering with increased ibuprofen. Bilayers were prepared with concentrations of: 0 mol% 10 mol% and 20 mol% ibuprofen. Only a lamellar peak is observed for pure DMPC. The observed diffuse scattering was attributed to lamellar diffuse scattering due to fluctuations and defect scattering as the result of a small fraction of bilayers not perfectly aligned on the substrate.31 For a membrane with 10 mol% ibuprofen, the defect scattering significantly increased indicative of a large fraction of misaligned bilayers. A cubic pattern is observed at 20 mol% ibuprofen. Intensities are shown on a logarithmic scale. |
What Are Cellular Membranes Made Of
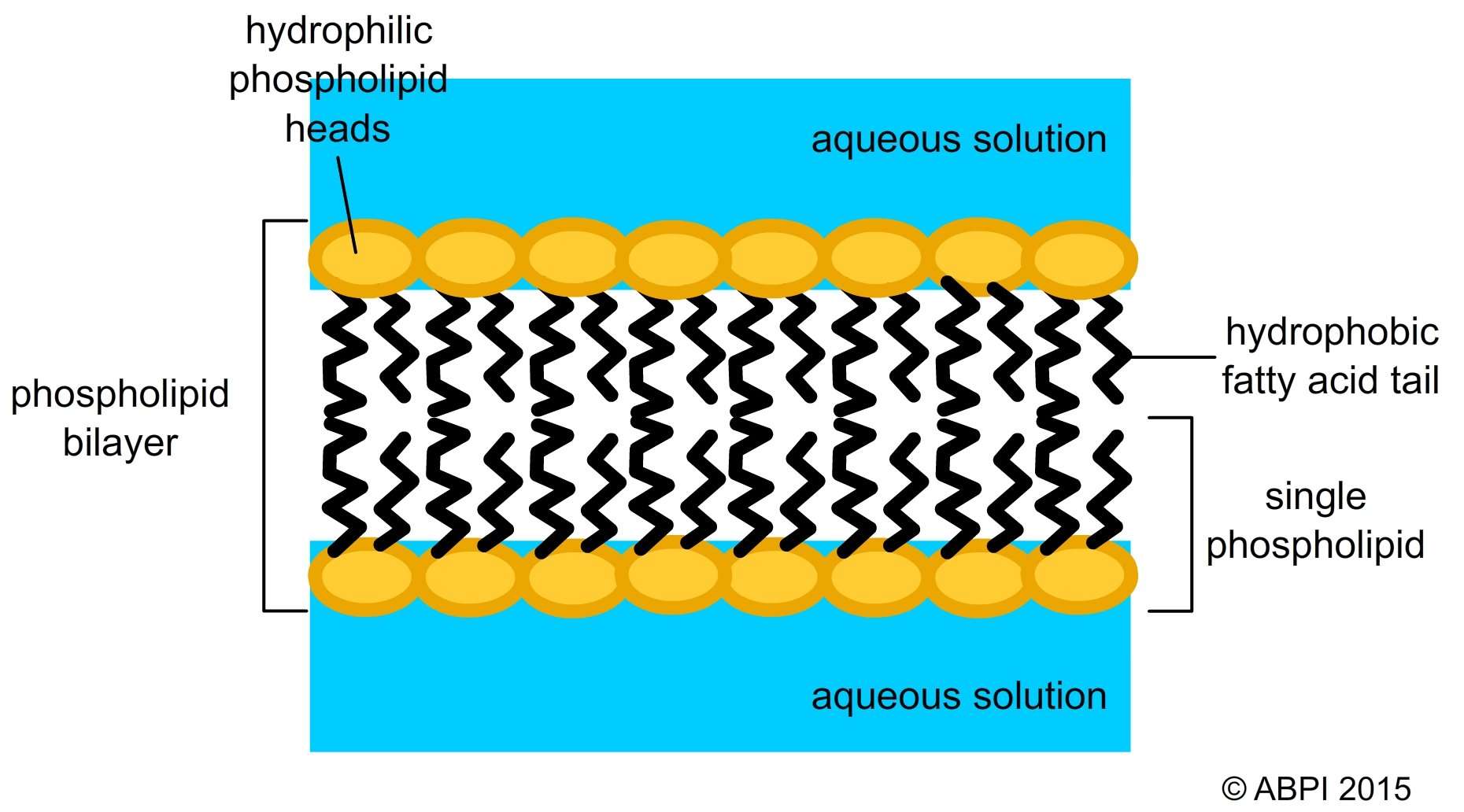
With few exceptions, cellular membranes including plasma membranes and internal membranes are made of glycerophospholipids, molecules composed of glycerol, a phosphate group, and two fatty acid chains. Glycerol is a three-carbon molecule that functions as the backbone of these membrane lipids. Within an individual glycerophospholipid, fatty acids are attached to the first and second carbons, and the phosphate group is attached to the third carbon of the glycerol backbone. Variable head groups are attached to the phosphate. Space-filling models of these molecules reveal their cylindrical shape, a geometry that allows glycerophospholipids to align side-by-side to form broad sheets .
Also Check: How Much Cholesterol In Pork Chops
Preparation Of The Multi
1,2-Dimysteroyl-sn-glycero-3-phosphocholine and cholesterol were obtained from Avanti Polar Lipids and individually dissolved in 1:1 mixtures of chloroform and tri-fluoro-ethanol . Ibuprofen was also dissolved in a mixture of 1:1 chloroform and TFE. The DMPC, cholesterol and ibuprofen solutions were then mixed in the appropriate ratios to achieve the desired membrane compositions for the experiment. All samples prepared for this study are listed in Table 2. Molecular representations of the components are depicted in Fig. 1.
Why Is It Important That The Phospholipid Is Both Hydrophobic And Hydrophilic
4.6/5hydrophilichydrophobicanswer here
The lipid bilayer is arranged in two layers of phospholipids with the hydrophilic heads forming the outer edges and the tails forming the interior. This is important because it allows the bilayer to select which molecules it will allow into and out of the cell.
Furthermore, how does hydrophilic and hydrophobic relate to the cell membrane? Cell membranes are made up of a double layer of these phospholipid molecules. This is because in water the hydrophilic heads will face the water while the hydrophobic tails will be in the center because they face away from the water. The phospholipid bilayer makes the membrane very stable but also allows flexibility.
Keeping this in consideration, why are tails of phospholipids hydrophobic?
Phospholipids are amphipathic molecules. This means that they have a hydrophilic, polar phosphate head and two hydrophobic fatty acid tails. These components of the phospholipids cause them to orientate themselves, so the phosphate head can interact with water and the fatty acid tails can’t, hence forming a bilayer.
What part of a phospholipid is hydrophilic?
A phospholipid consists of a head and a tail. The head of the molecule contains the phosphate group and is hydrophilic, meaning that it will dissolve in water. The tail of the molecule is made up of two fatty acids, which are hydrophobic and do not dissolve in water.
You May Like: Is Beef Bone Broth High In Cholesterol
Statin Solubility And Cardiovascular Outcomes
Statins inhibit cholesterol synthesis, thereby enhancing LDL clearance from the circulation. Since mevalonic acid is the precursor of numerous metabolites, HMG-CoA reductase inhibition potentially results in pleiotropic effects that may affect several tissue functions and modulate specific signal transduction pathways . These effects include anti-inflammatory and antioxidant activities, improvement in endothelial function, increased bioavailability of nitric oxide and delay in the progression of atherosclerotic plaques . All these vasculoprotective effects may account for a greater magnitude of and earlier time to cardiovascular benefit than apparently explained by changes in LDL cholesterol levels alone. However, leaving aside in vitro and experimental studies, it is not possible from available clinical evidence to isolate the potential benefits of pleiotropic effects of statins from those conferred by LDL cholesterol reduction.
Atheroprotective effects of statins. NO, nitric oxide LDL, low-density lipoprotein HDL, high-density lipoprotein.
One of the factors to be considered when evaluating outcomes of lipid-lowering treatment with statins is their solubility. Therefore, the role of both hydrophilic and lipophilic statins regarding beneficial pleiotropic effects and thus a possible improvement in cardiovascular primary or secondary prevention needs to be analysed further.
Which Part Of A Lipid Is Polar
Lipids and Phospholipids Each lipid molecule contains a hydrophilic region, also called a polar head region, and a hydrophobic, or nonpolar tail region. Figure %: Basic Lipid Structure The hydrophilic region is attracted to aqueous water conditions while the hydrophobic region is repelled from such conditions.
Recommended Reading: Are Mussels High In Cholesterol
What Is Hard Water Called
Hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Water hardness that is caused by calcium bicarbonate is known as temporary, because boiling converts the bicarbonate to the insoluble carbonate hardness from the other salts is called permanent.
Role Of Cholesterol In Lipid Bilayer
The lipid bilayer is a thin biological membrane that is made of two lipid layers. Each layer is built with phospholipids that contain a hydrophilic head and a hydrophobic tail. This structure is fundamental for the functioning of a cellular membrane.
Cholesterol is one of the lipid components that are present in the lipid bilayer. This waxy substance belongs to the steroid family and it is crucial not only for the functionality of the cellular membrane, but also for human existence as it serves as a precursor to synthesize many of the bodys compounds, such as steroids hormones, vitamin D, and bile acids.
Keep on reading to learn about the cell membrane lipid bilayer structure, formation, components, the role of cholesterol in the lipid bilayer, and how this waxy substance affects membrane fluidity. Everything easily explained to facilitate full comprehension.
Lipid Bilayer Structure
To start off, lets take a look at the lipid bilayer structure. Within the animals cell membrane, lipids constitute nearly 50% of its total mass. All of the lipids present in the cell membrane are amphipathic, which means that they possess a hydrophilic and a hydrophobic end.
The most predominant lipids are phospholipids, they have a hydrophilic head group and a hydrophobic tail, which are usually fatty acids.
The fatty acids tails can differ from each other in different aspects. One potential difference is their length, usually, they contain between 14 and 24 carbon atoms.
Fluid Mosaic Model
Don’t Miss: Is Bone Marrow High In Cholesterol
Cyclodextrins As A Tool To Study Membrane Rafts
Fig. 2.
Chemical structures of the three different types of cyclodextrin, alpha containing 6 glucopyranose units, beta containing 7 glucopyranose units and gamma containing 8 glucopyranose units. Representative images of the rings formed by the three different cycodextrins and the respective size of their pockets.
Do Hydrophobic And Hydrophilic Attract
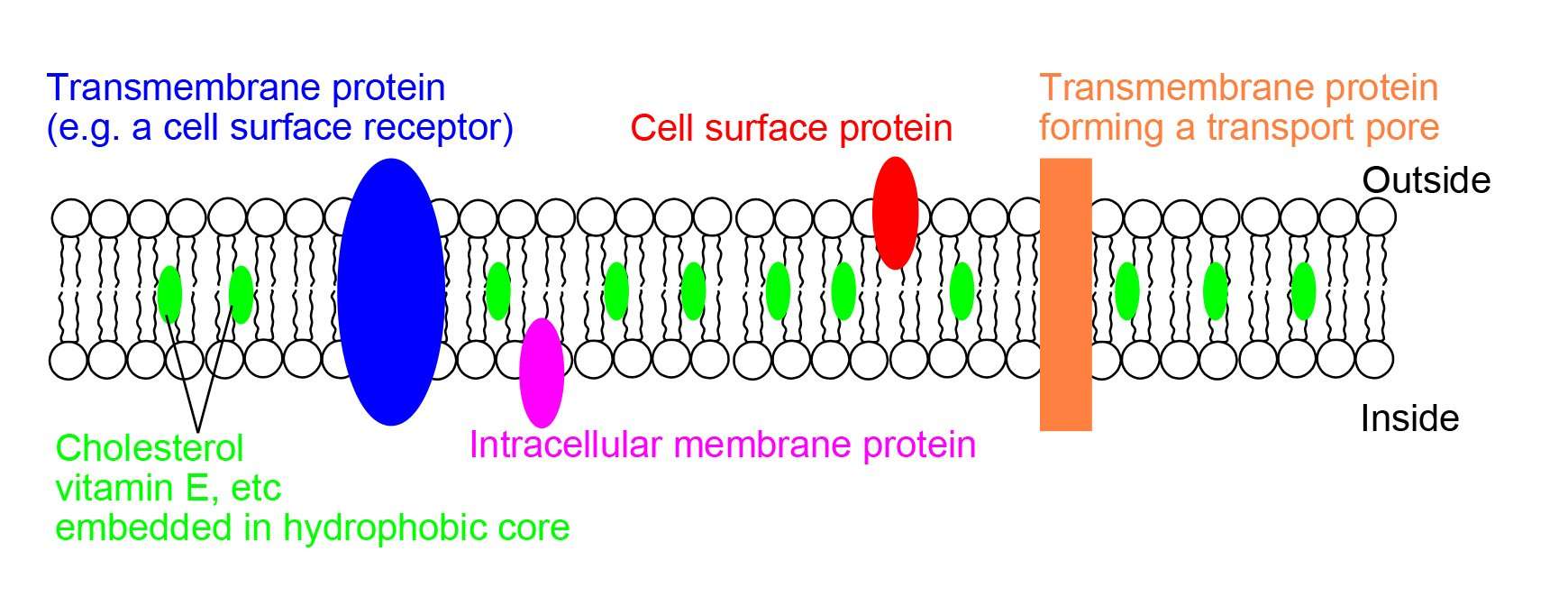
It is therefore erroneous to believe that only two hydrophobic entities attract each other when immersed in water: one hydrophobic and one hydrophilic entity usually also attract one another in water, albeit with a somewhat lower energy than is commonly seen with the attraction between two hydrophobic entities.
Read Also: Is Shrimp Bad For Your Cholesterol
The Interaction Of Ibuprofen With Membranes Containing Cholesterol
| Fig. 7 Out-of-plane diffraction for bilayers prepared with 20 mol% cholesterol and ibuprofen concentrations of: 0 mol%, 5 mol%, 20 mol%. |
| Fig. 8 Electron density profiles for DMPC membranes prepared with 20 mol% cholesterol and 20 mol% cholesterol with 5 mol% ibuprofen . The curves in are on an absolute scale, while the ibuprofen containing curve in was scaled to overlap the profile with that of a 20 mol% cholesterol-containing DMPC membrane . The difference between the scaled curve and the black curve is best described by two Gaussian profiles, which are labelled in . |
Explain How The Hydrophilic And Hydrophobic Properties Of Phospholipids Help To Maintain The Structure Of Cell Membranes 6m
| Integral proteins are embedded in the membrane/phospholipid bilayer peripheral proteins are on the surface of the membrane some integral proteins extend from one side ofthe membrane to the other hormone binding sites e.g. insulin enzymes e.g. sucrase / succinate dehydrogenase cell adhesion cell-to-cell communication recognition / antigenic markers / glycoproteins / contact inhibition Channels/pores for passive transport/facilitated diffusion pumps/carriers for active transport Receptors for neurotransmitters such as acetylcholine electron carriers e.g. electron transport chain of cellular respiration pigments | Membranes are surrounded by water hydrophilic molecules are attracted to water hydrophobic molecules are attracted to one another/repel water phospholipids are amphipathic/have a hydrophobic tail and a hydrophilic head tails are positioned away from water / heads are positioned towards water phospholipids have a hydrocarbon tail and a phosphate head phospholipid bilayer/ membranes self-assemble in water protein association with membrane is determined by hydrophobic interactions phospholipid bilayer is hydrophilic on the outside and hydrophobic on the inside |
You May Like: Is Shrimp Cholesterol Bad
The Suppression Of Cubic Phases By Cholesterol
Oriented membranes with ibuprofen concentrations less than 5 mol% formed lamellar phases, while samples with concentrations greater than 5 mol% could not be indexed by a single 1-dimensional lamellar phase and required a 3-dimensional cubic phase to index all peaks. The observed Bragg peaks for all samples in the cubic phase are consistent with either Im3m or Pn3m space groups, which are frequently observed in membrane systems.36,43,44 The peak, which we did not observe, is systematically absent for Im3m but not Pn3m, suggesting Im3m is the best candidate. Another frequently observed cubic phase, with space group Ia3d, did not describe the peaks as the reflection was observed.44,45
There is evidence that the impact of certain drugs on the lipid membrane is dependent on membrane composition. For example, negatively charged lipids have been shown to accelerate the binding of the antimicrobial peptide Lacticin Q.48 In addition, the anti-cancer drug Taxol has a different impact on saturated model membranes and unsaturated membranes.49 In a recent paper by Khajeh et al., molecular dynamics simulations were performed on membranes with cholesterol and ibuprofen32 to report that the permeation of ibuprofen across the membrane is decreased by an increased stiffness of the membrane caused by cholesterol.
Cholesterol And Membrane Rafts
Cholesterol displays a very important function as a component of cellular membranes, specially the cell plasma membrane where it is found in higher concentrations. Its positioning into the lipid bilayer and interaction with other lipids have a significant role in membrane fluidity together with other lipid components, such as the amount of sphingomyelin or the degree of saturation of the phospholipid acyl chains . Cholesterol fits most of its structure into the lipid bilayer and only the small hydroxyl group faces the external environment. As a consequence, its steroid rings are in close proximity and attracted to the hydrocarbon chains of neighboring lipids. This gives a condensing effect on the packing of lipids in cell membranes . However this effect seems to depend on the type of lipid it interacts with. As cholesterol hydrocarbon chain is rigid it tends to segregate together with fatty acids with saturated long acyl chains, especially sphingomyelin, leading to the formation of more compact liquid ordered and less fluid phases .
Also Check: Can Dehydration Skew A Cholesterol Test
How Do Lipids Affect The Body
Lipids play diverse roles in the normal functioning of the body: they serve as the structural building material of all membranes of cells and organelles. they provide energy for living organisms providing more than twice the energy content compared with carbohydrates and proteins on a weight basis.
Itioning Of Ibuprofen In Saturated Lipid Membranes With And Without Cholesterol
| Fig. 9 Measured difference in electron density with the addition of ibuprofen to DMPC membranes and calculated electron distributions of ibuprofen molecules. Three membrane bound states are fit to changes in electron density when 2 mol% ibuprofen is added to pure DMPC bilayers. The calculations take into account ibuprofen molecules, which extend into and are shared with neighbouring bilayers. Two embedded states are fit to the observed changes in electron density when 5 mol% ibuprofen is added to a membrane composed of DMPC + 20 mol% cholesterol. |
Three different membrane bound populations were observed when 2 mol% ibuprofen were added to the DMPC bilayers, as sketched in Fig. 9: a state in the hydrophobic membrane core, where the ibuprofen molecules align parallel to the lipid acyl chains 56% of ibuprofen molecules were found in this state 8% of ibuprofen molecules were observed at the interface between head groups-tail groups, and 36% of ibuprofen molecules were found attached to the membrane head group region, situated between the lipid head groups of two bilayers. At ibuprofen concentrations greater than 5 mol% , disruption of the lamellar membrane phase and the formation of a cubic lyotropic phase was observed.
Recommended Reading: Is Shrimp Bad For Your Cholesterol
Do Hydrophobic Or Hydrophilic Pass Through Membranes
The lipid bilayer is the main fabric of the membrane, and its structure creates a semipermeable membrane. The hydrophobic core impedes the diffusion of hydrophilic structures such as ions and polar molecules, but allows hydrophobic molecules, which can dissolve in the membrane, to cross it with ease.
Phospholipids And Biological Membranes
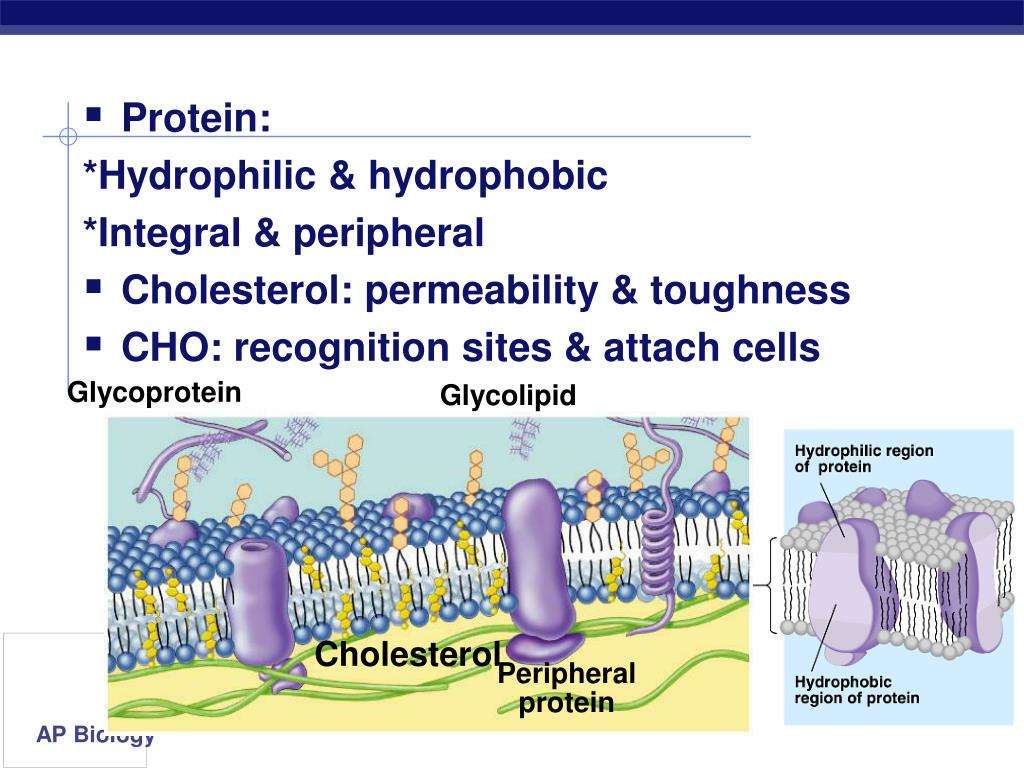
Figure 2. This illustration shows a phospholipid with two different fatty acids, one saturated and one unsaturated, bonded to the glycerol molecule. The unsaturated fatty acid has a slight kink in its structure due to the double bond.
Triglycerides are classified as simple lipids because they are formed from just two types of compounds: glycerol and fatty acids. In contrast, complex lipids contain at least one additional component, for example, a phosphate group or a carbohydrate moiety . Figure 2 depicts a typical phospholipid composed of two fatty acids linked to glycerol . The two fatty acid carbon chains may be both saturated, both unsaturated, or one of each. Instead of another fatty acid molecule , the third binding position on the glycerol molecule is occupied by a modified phosphate group.
Figure 3. Phospholipids tend to arrange themselves in aqueous solution forming liposomes, micelles, or lipid bilayer sheets.
Also Check: Does Feta Cheese Affect Cholesterol
Hydrophilic Vs Lipophilic Statins
Since the introduction of lovastatin in 1987 as the first 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor approved for human therapy, statins have become the most widely used lipid-lowering drugs with proven effect in cardiovascular disease prevention in different clinical settings . Statins have been classified in 3 categories based on their potency and efficacy in lowering low-density lipoprotein cholesterol concentrations. First-generation statins included lovastatin, pravastatin and fluvastatin, simvastatin and atorvastatin belong to the second generation, and rosuvastatin and pitavastatin to the third.
Chemical structure of hydrophilic and lipophilic statins.
Why Are Phospholipids Hydrophobic And Hydrophilic
4/5Phospholipidshydrophilichydrophobicfull answer
The phospholipids in the plasma membrane are arranged in two layers, called a phospholipid bilayer. As shown in the Figure below, each phospholipid molecule has a head and two tails. The head loves water and the tails hate water .
Similarly, is the cell membrane hydrophobic or hydrophilic? The cell membrane consists primarily of a thin layer of amphipathic phospholipids that spontaneously arrange so that the hydrophobic “tail” regions are isolated from the surrounding water while the hydrophilic “head” regions interact with the intracellular and extracellular faces of the resulting bilayer.
Also question is, why are hydrophilic heads important?
The lipid bilayer is arranged in two layers of phospholipids with the hydrophilic heads forming the outer edges and the tails forming the interior. This is important because it allows the bilayer to select which molecules it will allow into and out of the cell.
Why are lipids hydrophobic?
Lipids are mainly composed of carbon and hydrogen atoms, and this hydrophobic nature of lipids is driven by the bonds between these many carbons and hydrogens. Thus, long chains of carbon-hydrogens bonds form a nonpolar molecule.
Also Check: Does Feta Cheese Have Cholesterol