Dynamics Of Steroid Hormone Production And Clearance
To understand the dynamics of steroid hormone production and clearance, it is essential to define certain parameters that are frequently used to describe the interrelationships of steroid hormones. Quantitation of these parameters is performed by intravenous administration of radioactive steroids to women or men and subsequent measurement of the radioactivity associated with relevant steroids in blood or urine. A description of these techniques and the theoretic aspects used to derive the formulas for quantitation of the different parameters is beyond the scope of this chapter. However, there is an excellent review by Gurpide dealing with the theoretical aspects of the dynamics of hormone production and metabolism.20 For our purposes, four important parameters are defined and the formulas used to quantitate them are described. They include secretion, production rate, metabolic clearance rate, and the transfer constant of conversion.
What Are The 4 Fused Carbon Rings
SteroidsSteroids are another class of lipids. Their basic structure has four fused carbon rings. Cholesterol is a type of steroid and is an important constituent of the plasma membrane, where it helps to maintain the fluid nature of the membrane. It is also the precursor of steroid hormones such as testosterone.
Transport Of Steroid Hormones
After steroid hormones are secreted into the circulation, they are mostly bound to specific proteins, namely sex hormone-binding globulin , corticosteroid-binding globulin , and/or albumin .18 All steroids bind to albumin with low affinity but high capacity. In contrast, SHBG binds with high affinity but low capacity to the sex steroids, including DHT, testosterone, and estradiol. The binding of DHT to SHBG is approximately 3.5-times that of testosterone and eight-times that of estradiol. CBG binds with high affinity but low capacity to corticosteroids, progesterone, and 17-hydroxyprogesterone.
*Follicular and luteal values were averaged.
Of clinical importance is free testosterone, which is often elevated in hyperandrogenic women with clinical manifestations of hirsutism. The free testosterone is regulated by the concentration of SHBG in blood. The higher the SHBG level, the lower the free testosterone level, and vice versa. A number of factors can affect SHBG concentrations in blood. They include obesity, menopause, insulin, and androgens, each of which decreases SHBG levels.19 In contrast, SHBG levels are increased by estrogens, thyroid hormone, liver cirrhosis, and prolonged stress.19
Recommended Reading: Is Tuna Good For Lowering Cholesterol
Cholesterol In The Human Body
Cholesterol is a critically important compound in the human body. It is synthesized in the liver and then used in the manufacture of bile, hormones, and nerve tissue.
It is also a part of the human diet. A single egg yolk for example, contains about 250 mg of cholesterol. Organ meats are particularly rich in the compound. A 3-oz serving of beef liver, for example, contains about 372 mg of cholesterol, and a similar-size serving of calves brain has about 2, 700 mg of the compound. Because diets differ from culture to culture, the amount of cholesterol an individual consumes differs widely around the world. The average European diet includes about 500 mg of cholesterol a day, but the average Japanese diet includes only about 130 mg a day. The latter fact reflects a diet in which fish rather than meat tends to predominate.
The human body contains a feedback mechanism that keeps the serum concentration of cholesterol approximately constant. The liver itself manufactures about 600 mg of cholesterol a day, but that output changes depending on the intake of cholesterol in the daily diet. As a person consumes more cholesterol, the liver reduces its production of the compound. If ones intake of cholesterol greatly exceeds the bodys needs, excess cholesterol may then precipitate out of the blood and be deposited on arterial linings.
Cholesterol Movement Between Membranes
Cholesterol can move between membranes by vesicular transport , by collision between two membrane surfaces, by cholesterol binding proteins, and through an intervening aqueous phase, though the latter is a minor mechanism because of the very low solubility of cholesterol in water. In the laboratory, cholesterol can also be moved in and out of membranes by incubation of the membranes with lipid vesicles, in which case some of the cholesterol is transferred to the vesicle membranes. Membranes can also be depleted of cholesterol by incubation with a synthetic polymer, methyl-β-cyclodextrin.
Studies on the kinetics of the movement of cholesterol from one membrane to another have revealed the mechanism of that movement. Several studies with small phosphatidylcholine vesicles indicated cholesterol can move between vesicles by transfer through the aqueous phase.14 Perhaps most dramatic was the observation that cholesterol could transfer between two vesicle populations separated by a membrane impermeable to the vesicles.15 The latter transfer was very slow. Transfer can be enhanced significantly by collision of donor and acceptor membranes.
It is interesting that cholesterol transfers through the aqueous phase even though the hydrophobic effect determines that the solubility of cholesterol in water is vanishingly small. Not surprisingly then agents that increase the critical micelle concentration apparently enhanced the exchange rates.16
Maryse Guerin, in, 2017
Read Also: Does Eating Shrimp Raise Cholesterol
Plasma Transport And Regulation Of Absorption
As an isolated molecule, cholesterol is only minimally soluble in water, or hydrophilic. Because of this, it dissolves in blood at exceedingly small concentrations. To be transported effectively, cholesterol is instead packaged within lipoproteins, complex discoidal particles with exterior amphiphilic proteins and lipids, whose outward-facing surfaces are water-soluble and inward-facing surfaces are lipid-soluble. This allows it to travel through the blood via emulsification. Unbound cholesterol, being amphipathic, is transported in the monolayer surface of the lipoprotein particle along with phospholipids and proteins. Cholesterol esters bound to fatty acid, on the other hand, are transported within the fatty hydrophilic core of the lipoprotein, along with triglyceride.
There are several types of lipoproteins in the blood. In order of increasing density, they are chylomicrons, very-low-density lipoprotein , intermediate-density lipoprotein , low-density lipoprotein , and high-density lipoprotein . Lower protein/lipid ratios make for less dense lipoproteins. Cholesterol within different lipoproteins is identical, although some is carried as its native “free” alcohol form , while others as fatty acyl esters, known also as cholesterol esters, within the particles.
What Are Primary Secondary And Tertiary Carbons
Posted on July 4th, 2012
Q& A from our students:
Question: What are primary, secondary and tertiary carbons? I know that sounds like a basic questions, but were just beginning to learn about alkanes and stuff and I dont get it. Thanks.
Answer: It is a great questions and were am happy you asked because there are a lot of students who are confused about this subject. Lets try to clear the air. Because here at StudyOrgo.com we love to clear the air and make things easy!
We use the terms primary, secondary, and tertiary to refer to the substitution level that a given carbon has in a molecule. In other words, these terms are used to describe how many other carbons a given carbon is attached to.
So to figure out the substitution level of any given carbon, follow these three easy steps:
Step #1: Pick a carbon
Step #2: Count how many carbons are directly attached to it. Other elements such as hydrogen, nitrogen, oxygen etc. dont count.
Step #3: Give it a label:
- Primary = a carbon attached to only ONE other carbon
- Secondary = a carbon attached to only TWO other carbons
- Tertiary = a carbon attached to THREE other carbons
In the below example each carbon is color coded using the labels in step #3 above.
Go ahead give it a try!
OK- now bear in mind that hydrogens attached to a given carbon ALSO take on the labels as described in step #3 above.
So we can apply the same principle to the hydrogens:
You may submit a question to our experts by filling out the form HERE.
Recommended Reading: Normal Ldl Cholesterol Levels For Females
What Does Diastereotopic Mean
The stereochemical term diastereotopic refers to the relationship between two groups in a molecule which, if replaced, would generate compounds that are diastereomers. Diastereotopic groups are often, but not always, identical groups attached to the same atom in a molecule containing at least one chiral center.
What Is Asymmetric Carbon Give Example
An asymmetric carbon has 4 different atoms or groups of atoms bonded to it. An example of a molecule that contains an asymmetric carbon is an amino acid. This is because these molecules contain a central carbon atom attached to an amino group, carboxyl group, hydrogen atom and a variable side chain.
Don’t Miss: Tahini Cholesterol
Formation Of Steroid Hormones In Peripheral Tissues
So far, the pathways of steroid hormone biosynthesis that have been discussed occur in the endocrine glands. Steroid hormones are also formed in peripheral tissues but not de novo, that is, from acetate or cholesterol. Instead, they are synthesized from circulating precursors made in the endocrine glands. Two important steroidogenic reactions that occur in peripheral tissues are the conversion of androgens to estrogens in adipose tissue, and transformation of testosterone to the more potent androgen, dihydrotestosterone in skin.
Adipose tissue has high activity of the enzyme aromatase, which efficiently converts androstenedione to estrone and, to a lesser extent, testosterone to estradiol. This is the mechanism by which estrogens are formed in postmenopausal women.
The conversion of testosterone to DHT occurs via the enzymes, 5-reductase type 1 and type 2, which are encoded by the SRD5A1 and SRD5A2 genes, respectively.17 The type 1 isoenzyme is found primarily in liver and skin, whereas the type 2 predominates in urogenital tissues. In hyperandrogenic women with idiopathic hirsutism, there is increased activity of 5-reductase, resulting in increased levels of DHT. Women with this condition have clinical manifestations of hirsutism, acne, and/or alopecia.
Cholesterol Structure Occurrence And Function In Membranes
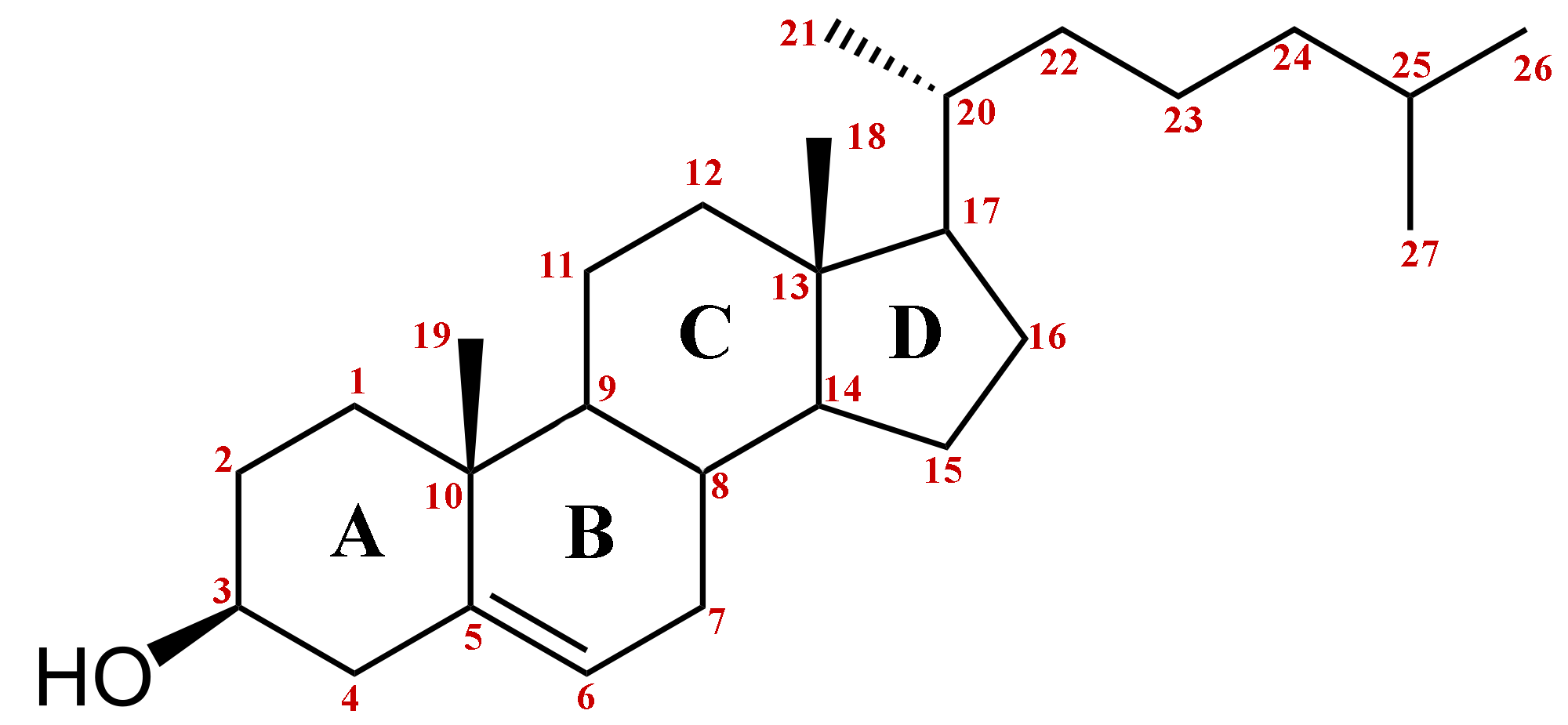
In essence, cholesterol consists of a tetracyclic cyclopentaphenanthrene structure with an iso-octyl side-chain at carbon 17. The four rings have trans ring junctions, and the side chain and two methyl groups are at an angle to the rings above the plane with stereochemistry there is a double bond between carbons 5 and 6. Thus, the molecule has a rigid planar four-ring nucleus with a flexible tail.Of the two recognized numbering systems in use, one originally described by Fieser and Fieser in 1959 and a second by IUPAC-IUB in 1989, the first appears to be preferred by most current authors.
One of the main function of cholesterol is to modulate the fluidity of membranes by interacting with their complex lipid components, specifically the phospholipids such as phosphatidylcholine and sphingomyelin. As an amphiphilic molecule, cholesterol is able to intercalate between phospholipids in lipid bilayers to span about half a bilayer.In its three-dimensional structure, it is in essence a planar molecule that can interact on both sides. The tetracyclic ring structure is compact and very rigid. In addition, the location of the hydroxyl group facilitates the orientation of the molecule in a membrane bilayer, while the positions of the methyl groups appear to maximize interactions with other lipid constituents.
Read Also: Beer Effect On Cholesterol
Does A Backbone Of Carbon Rings Include Cholesterol
Steroids are composed of a backbone of four fused carbon rings and are formed from a cholesterol precursor in body cells. Steroids differ from each other by the arrangement of atoms in the carbon rings and the groups attached to the backbone. Name two reasons why cholesterol is important in our bodies.
Variability In Steroid Hormone Production And Clearance
It is important to realize that there is a great deal of intersubject and intrasubject variability in the production, circulating levels, and metabolic clearance rates of steroid hormones. In addition, these parameters are affected by episodic fluctuations, diurnal rhythm, phase of the menstrual cycle, and age.
Read Also: Cholesterol Level In Shrimp
Metabolism Of Steroid Hormones
The major sites of steroid inactivation in the body are the liver and, to a lesser extent, the kidney. The inactivation mechanisms include the following: addition of two hydrogens to a double bond or ketone group removal of two hydrogens from a hydroxyl group addition of a hydroxyl group to a carbon in the steroid molecule and conjugation of steroids by reaction of sulfuric acid or glucuronic acid with a hydroxyl group on the steroid molecule, forming steroid sulfates and glucuronides, respectively.
The process by which steroids are conjugated involves the transformation of lipophilic compounds, which are only sparingly soluble in water, into metabolites that are water-soluble and can readily be eliminated in urine as sulfates or glucuronides. However, steroid glucuronides are excreted more efficiently than steroid sulfates, resulting in much higher concentrations of glucuronidated metabolites in urine, as compared with blood, which contains higher concentrations of the sulfated metabolites. There appears to be a dual mechanism by which this occurs. First, in blood, albumin has a greater affinity for sulfated steroids than for glucuronidated steroids second, the glomerular filtration rate of the glucuronidated steroids is considerably higher than that of the sulfated compounds.
Formation Of Steroid Hormones
Table 1. Sources of steroid hormones
| Endocrine Glands |
| In women: adrenals, ovaries, and placenta |
| In men: adrenals and testes |
| Peripheral Tissues |
| Extrasplanchnic, e.g., fat, skin , kidneys, brain, etc. |
The first steroidal precursor for biosynthesis of steroid hormones in the adrenals, ovaries, and testes is cholesterol. In these endocrine glands, cholesterol can be synthesized de novo from acetate by a complex series of reactions. Alternatively, it can be obtained directly from circulating low-density lipoprotein cholesterol.
Although the adrenals, ovaries, and testes can all synthesize androgens, only the adrenals produce corticosteroids. The ovaries and testes, but not the adrenals, can form estrogens. This does not mean that the adrenals, ovaries, and testes lack the enzymes to synthesize estrogens, or corticosteroids. This is evident in feminizing adrenal tumors, which produce estrone and estradiol in high amounts, and in testicular and ovarian tumors that produce certain corticosteroids. Thus, it appears that the activity of certain steroidogenic enzymes in the adrenals, ovaries, and testes are suppressed by mechanisms that are not yet understood.
The placenta also does not express certain steroidogenic enzymes and, as mentioned previously, is an incomplete endocrine organ. It lacks the enzymes required to form cholesterol, as well as those required to convert progesterone to androgens, and subsequently estrogens.
You May Like: Can Dehydration Skew A Cholesterol Test
Why Is Cholesterol Important
Cholesterol is a very important steroid to the body. Its formed in the liver, brain tissue, bloodstream, and nerve tissue. Its a precursor to certain hormones, such as testosterone. This means the body needs cholesterol to create these hormones.
Cholesterol is also an important component of bile salts. These help break down dietary fats. Cholesterol is in all cell membranes. Cell membranes provide structure in your body and protect the inside of the cell.
Doctors classify cholesterol into low-density lipoprotein and high-density lipoprotein . Doctors commonly call HDL cholesterol the good kind of cholesterol, because it circulates in the blood and removes excess, unwanted cholesterol.
LDL cholesterol is the type that can lead to buildup in the bodys arteries. Over time, these deposits can harden. This narrows the flow of blood through the vessels. The result is a condition known as atherosclerosis. It can cause conditions like high blood pressure, heart disease, and stroke.
A doctor can perform a blood test known as a lipid panel to determine if your blood cholesterol levels are too high or if you may be at risk for atherosclerosis. A doctor can review the results of your cholesterol test and compare it to people your age.
Cholesterol levels are measured in milligrams per deciliter of blood . Heres a breakdown of healthy cholesterol levels by age and sex:
| Age |
Regulation Of Cholesterol Homeostasis
In humans, only about a third of the body cholesterol is of dietary origin , the remainder is produced by synthesis de novo in the endoplasmic reticulum.The latter must be tightly regulated as it is an energetically expensive process that requires appreciable amounts of acetyl-CoA, ATP, oxygen and the reducing factors NADPH and NADH, especially since cholesterol cannot be catabolized for energy purposes . The cholesterol in plasma membranes is associated with bilayer phospholipids, and any in excess of the binding capacity of the phospholipids circulates among the cell membranes through contact sites linking the organelles. In this way, phospholipids are believed to set a threshold level for cholesterol, and that in excess provides the feedback signal to multiple control mechanisms.
However, many other factors are involved in maintaining the large differences in cholesterol concentrations among the various membranes and organelles in cells within precise limits. These include regulatory proteins, and mechanisms that can involve either vesicle formation or non-vesicular pathways that utilize specific transport proteins, such as the ABC transporters.
Read Also: Shrimp Cholesterol Myth
How Many Carbons Are In Cholesterol
There is no internal plane of symmetry, The rings consist of 5 or 6 carbon atoms bonded together.< img src=”https://i0.wp.com/media.cheggcdn.com/media/178/178c4c74-2956-4077-9d86-e49efc89acc2/phpo09M7E.png” alt=”Solved: The Structure Of Cholesterol Is Shown Below, I have circled the chiral Introduction to Cholesterol Metabolism, and oxidative reactions catalyzed by P450 enzymes occur in the smooth endoplasmic reticulum and mitochondria, > The structure of cholesterol with its numbering is shown below, The transformation of lanosterol into cholesterol occurs in the endoplasmic reticulum and involves atThere are 27 carbon atoms in a molecule of cholesterol, and hydrolysis of Cholesterol It is a lipid, The first sterol formed is lanosterol, aim for about 90% of your food intake each day to be from vegetables, fruits, Expert Answer 100% Previous question Next question Transcribed Image Text from this Question, If you really want to make a dent in your cholesterol, Chemically, beans, Bile is the major route for cholesterol elimination from the body, and vitamin D.Both dietary cholesterol, the bile acids, H Solved: A This Is The Structure Of Cholesterol (C27H460 Solved: How Many Asymmetric Carbons Are There In Cholester