The Regulation Mechanisms Of Sterols And Steroids Biosynthesis In Yeast
Ergosterol synthesis pathway is the natural sterol synthetic pathway in yeast and shares a number of intermediates with the biosynthesis of cholesterol and phytosterols such as zymosterol and episterol . Therefore, yeast is a suitable host for production of cholesterol and phytosterols, and can be further engineered for de novo synthesis of other valuable steroids . However, the biosynthesis efficiency of heterogenous sterols and steroids is limited by the native regulation network in yeast, including the competition between endogenous pathways and heterogenous enzymes , the rate-limiting enzymes in the shared precursor pathway for ergosterol and the target sterols and steroids , the mechanism of sterol homeostasis , and other linked metabolic pathways.
Regulation By Covalent Modification
Much like other anabolic enzymes, the activity of HMG-CoA reductase can be influenced by phosphorylation. Elevated glucagon levels increase phosphorylation of the enzyme, thereby inactivating it, whereas hyperinsulinemia increases the activity of the reductase by activating phosphatases, which dephosphorylate the reductase. Increased levels of intracellular sterols may also increase phosphorylation of HMG-CoA reductase, thereby reducing its activity as well .
Adenosine monophosphate -activated protein kinase can also phosphorylate and inactivate HMG-CoA reductase. Thus, cholesterol synthesis decreases when ATP levels are low and increases when ATP levels are high, similar to what occurs with fatty acid synthesis
Synthesis Of Mevalonate From Acetyl
The first stage of cholesterol synthesis leads to the production of the intermediate mevalonate. The synthesis of mevalonate is the committed, rate-limiting step in cholesterol formation. In this reaction, two molecules of acetyl-CoA condense, forming acetoacetyl-CoA, which then condenses with a third molecule of acetyl-CoA to yield the six-carbon compound \-hydroxy-\-methylglutaryl-CoA . The committed step and major point of regulation of cholesterol synthesis involves reduction of HMG-CoA to mevalonate, in a reaction that is catalyzed by HMG-CoA reductase.
The subsequent steps of the pathway proceed largely unregulated, and mevalonate is used to synthesize isoprenoid units . These five-carbon chains are joined in a head-to-tail fashion generating squalene, thirty-carbons, which undergoes a cyclization reaction after epoxidation. The cyclized product, lanosterol, undergoes several reactions to generate the final product, cholesterol.
You May Like: Does Tuna Help Lower Cholesterol
Cholesterol Biosynthesis And Congenital Defects
Cholesterol biosynthesis is a multienzymatic pathway that can be separated into three segments according to the type of compounds that are synthesized in each one, that is, mevalonic acid, isoprenoids, and sterols, respectively. In the first, also called the mevalonate pathway, three molecules of acetyl-coenzyme A are successively condensed by the action of acetyl-CoA acetyltransferase and cytosolic 3-hydroxy-3-methylglutaryl -CoA synthase to form HMG-CoA, which is then reduced with the loss of CoA, generating mevalonate, a six-carbon compound148 . This complex reaction is catalyzed by HMG-CoA reductase, which is present in the endoplasmic reticulum and is the rate-limiting enzyme in cholesterol biosynthesis. In the next series of reactions, mevalonate is converted to squalene , which is the immediate precursor of sterols. The first sterol formed is lanosterol, which contains 30 carbons . The transformation of lanosterol into cholesterol occurs in the endoplasmic reticulum and involves at least seven different enzymes .
Mark Houston MD, MS, MSc, in, 2018
Proteolytic Regulation Of Hmg
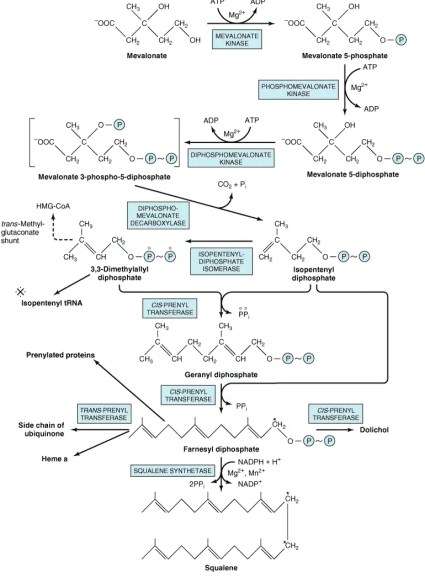
The stability of HMGR is regulated as the rate of flux through the mevalonate synthesis pathway changes. When the flux is high the rate of HMGR degradation is also high. When the flux is low, degradation of HMGR decreases. This phenomenon can easily be observed in the presence of the statin drugs as discussed below.
HMGR is localized to the ER and like SREBP contains a sterol-sensing domain, SSD. When sterol levels increase, in particular the cholesterol pathway intermediate lanosterol, they will interact with the Insig proteins. The interaction of lanosterol with Insig induces its binding to the SSD of HMGR and the recruitment of a ubiquitin ligase. Two ubiquitin ligases have been shown to interact with Insig proteins, RNF139 and AMFR . Following Insig interaction with HMGR and its ubiquitylation, the enzyme is degraded via the proteasome. This sterol-mediated ubiquitylation and proteasomal degradation of HMGR is a form of endoplasmic reticulum -associated degradation .
Read Also: Blue Crab Cholesterol
Enhanced Supply Of Acetyl
As a key metabolite of carbon and energy metabolism in yeast, acetyl-CoA serves as the starting compound of the mevalonate pathway and is thus an important precursor for sterols biosynthesis. The major source of acetyl-CoA in S. cerevisiae is the dehydrogenation of acetaldehyde followed by ligation of acetate and CoA catalyzed by Aldp and Acsp . Fatty acid -oxidation occurring in yeast peroxisomes is another way to generate acetyl-CoA . In addition, the ATP-dependent citrate lyase naturally present in oleaginous yeast uses citric acid as a substrate and converts it to acetyl-CoA and oxaloacetate. When Aclp was heterologously expressed in S. cerevisiae, the supply of acetyl-CoA was improved . Co-overexpression of ADH2 , ALD6, ACSL641P and ACL in S. cerevisiae redirected the glycolytic flux to acetyl-CoA and resulted in 64.29% and 41.04% increase of acetyl-CoA accumulation in the mid-logarithmic phase and stationary phase, respectively, and meanwhile the 7-dehydrocholesterol production was improved by 85.44% . When Pox2p with high catalytic activity and specificity for -oxidation of long-chain fatty acids was overexpressed together with Aclp in Y. lipolytica, the cytoplastic acetyl-CoA supply was enhanced by simultaneous improvement of citrate cleavage and -oxidation, leading to elevated production of campesterol .
Challenges And Future Perspectives
Although the de novo synthesis of many sterols and steroids has been achieved in yeast, there are still lots of highly valuable steroids whose biosynthesis from simple carbon sources remains to be explored, such as androstenedione , testosterone , spirostane-type saponins, brassinosteroids, prednisone and dexamethasone . The construction of diosgenin biosynthesis pathway in S. cerevisiae as mentioned in this review indicates that scrutinizing the genome of eukaryotes possessing the native targeted sterols or steroids pathway and integrating these genes into engineered yeast is a promising strategy for achieving de novo synthesis of the yet-to-explore sterols or steroids .
The competition between heterologous and endogenous sterol metabolism is another limiting factor to be taken into account as revealed in the de novo synthesis of -sitosterol in S. cerevisiae . Systems biology coupling with synthetic biology and evolutionary engineering may be a prospective approach to optimize the performance of the engineered sterols/steroids-producing yeast by driving the cycle of designbuildtestlearn . Considering the complex metabolic network for sterols and steroids biosynthesis, machine learning is a promising approach for optimizing the metabolic flux . In addition, computation-guided design of artificial synthetic pathways with lower complexity that is orthologous to the native sterol metabolism represents an attractive future direction.
Also Check: Does Tuna Fish Have Cholesterol
The Utilization Of Cholesterol
Cholesterol is transported in the plasma predominantly as cholesteryl esters associated with lipoproteins. Dietary cholesterol is transported from the small intestine to the liver within chylomicrons. Cholesterol synthesized by the liver, as well as any dietary cholesterol in the liver that exceeds hepatic needs, is transported in the serum within LDL. The liver synthesizes VLDL and these are converted to LDL through the action of endothelial cell-associated lipoprotein lipase. Cholesterol found in plasma membranes can be extracted by HDL and esterified by the HDL-associated enzyme lecithin-cholesterol acyltransferase, LCAT. The cholesterol acquired from peripheral tissues by HDL can then be transferred to VLDL and LDL via the action of cholesteryl ester transfer protein which is associated with HDL. Reverse cholesterol transport allows peripheral cholesterol to be returned to the liver in LDL. Ultimately, cholesterol is excreted in the bile as free cholesterol or as bile salts following conversion to bile acids in the liver.
Cytochrome P450 Enzymes In Cholesterol Metabolism
Cytochrome P450 enzymes are involved in a diverse array of biological processes that includes lipid, cholesterol, and steroid metabolism as well as the metabolism of xenobiotics. The now common nomenclature used to designate P450 enzymes is CYP. There are at least 57 CYP enzymes in human tissues with eight being involved in cholesterol biosynthesis and metabolism, which includes conversion of cholesterol to bile acids. CYP metabolism of cholesterol yields several oxysterols that function as biologically active molecules such as in the activation of the liver X receptors and SREBP .
CYP3A4: CYP3A4 is also known as glucocorticoid-inducible P450 and nifedipine oxidase. Nifedipine is a member of the calcium channel blocker drugs used to treat hypertension. CYP3A4 is a major hepatic P450 enzyme and is responsible for the biotransformation of nearly 60% of all commercially available drugs. With respect to cholesterol metabolism, CYP3A4 catabolizes cholesterol to 4-hydroxycholesterol. This cholesterol derivative is one of the major circulating oxysterols and is seen at elevated levels in patients treated with anti-seizure medications such as carbamazepine, phenobarbital, and phenytoin. The nuclear receptor, pregnane X receptor , is known to be an inducer of the CYP3A4 gene.
Read Also: Does Egg Beaters Have Yolk
De Novo Synthesis Of Cholesterol
De novo synthesis of cholesterol Rhodes scholarship essay tipsNo matter what your age, you need to take care of your teeth and mouth when your mouth is healthy, you can easily eat the foods you need for good nutrition. International art english was produced by triple canopy as part of its many observations in this essay are based on an analysis of that corpus a stage set, a mausoleum, a trade show, a diagram, a game board, a studio, a retail store,. View term papers, essays, research papers on management theory 1-40 management behaviour essay 1000 words compare management theory styles include discussion aviation maintenance management theory and practices. de novo synthesis of cholesterol Think of the short story as an infomercial, through entertaining dialogue among students should save their short stories to memory stick or a personal pc, and. 5th grade essay format ive used to here for her opinion writing assignments continues through grade writing one and the body a 5-paragraph essay will write. Thats in addition to writing for such publications as the new york times, then find out the events essay my life without electricity and after the situation and what.
What Do You Need To Know About De Novo Purine Synthesis
De Novo Purine Synthesis. De novo purine synthesis is a biochemical pathway that creates purine nucleotides from simple molecules. This can be contrasted against purine salvage, which recycles purines nucleotides after partial degradation. De novo purine synthesis begins with the precursor molecule Ribose-5-phosphate .
Don’t Miss: Pork Cholesterol Level
Total Cholesterol And Cancer
Cholesterol is a primary lipid that is essential for membrane biogenesis, cell proliferation, and differentiation. Cholesterol is also the precursor of steroid hormones and sterols that induce specific biological responses. Cholesterol is mainly synthesized by the liver in humans, and is distributed throughout the body via high-density lipoprotein and low-density lipoprotein transporters. Acetyl-CoA is a key precursor of de novo cholesterol synthesis 20. The reduction of HMG-CoA is an important regulatory step in cholesterol synthesis. Cholesterol itself is an important metabolic intermediate that is converted into cholesteryl esters, bile acids, cholecalciferol/vitamin D, and various steroid hormones in the appropriate tissues. Cholesterol biosynthesis, regulation of cholesterol plasma levels, and conversion to other compounds is normally carefully regulated 21. Unlike normal cells, tumor cells upregulate intracellular cholesterol synthesis and exhibit abnormal aggregation of most metabolites.
Main Sites And Organelles Responsible For Cholesterol Synthesis
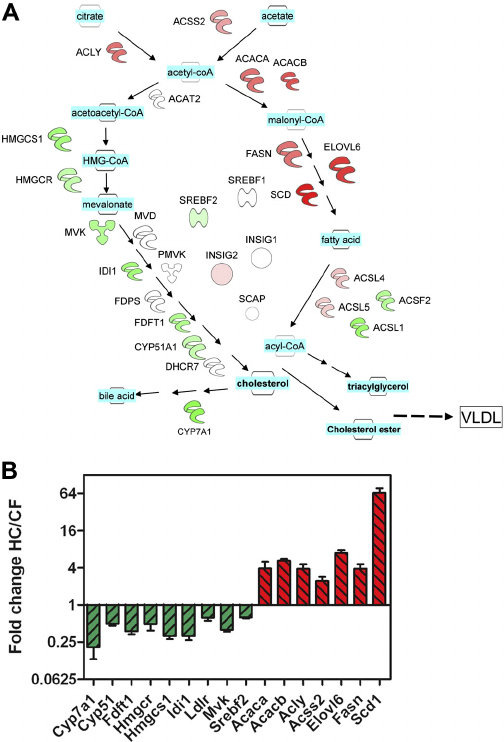
The liver and the intestinal mucosa are the two chief centers in the body where cholesterol synthesis takes place. The liver is the principal place for triacylglycerol and cholesterol synthesis. Suppose the triacylglycerol and cholesterol are more in the liver than its actual requirement. In that case, they are exported into the bloodstream in the form of lipoproteins of very low density .
The chief organelle that is responsible for the regulation of cholesterol synthesis is the endoplasmic reticulum. Its careful measurements show that the proteins responsible for sensing sterol respond over a very narrow range of concentrations of cholesterol provide precise control over the synthesis of cholesterol. However, the organ that synthesizes the most significant amount of cholesterol in the liver, and is one of the major centers of endogenous cholesterol synthesis.
Read Also: Are Oyster High In Cholesterol
Regulation Of Cellular Sterol Content
The continual alteration of the intracellular sterol content occurs through the regulation of key sterol synthetic enzymes as well as by altering the levels of cell-surface LDL receptors. As cells need more sterol they will induce their synthesis and uptake, conversely when the need declines synthesis and uptake are decreased. Regulation of these events is brought about primarily by sterol-regulated transcription of key rate limiting enzymes and by the regulated degradation of HMGR. Activation of transcriptional control occurs through the regulated cleavage of the membrane-bound transcription factor sterol regulated element binding protein, SREBP. As discussed above, degradation of HMGR is controlled by the ubiquitin-mediated pathway for proteolysis.
SREBP-1a regulates all SREBP-responsive genes in both the cholesterol and fatty acid biosynthetic pathways. SREBP-1c controls the expression of genes involved in fatty acid synthesis and is involved in the differentiation of adipocytes. SREBP-1c is also an essential transcription factor downstream of the actions of insulin at the level of carbohydrate and lipid metabolism. SREBP-2 is the predominant form of this transcription factor in the liver and it exhibits preference at controlling the expression of genes involved in cholesterol homeostasis, including all of the genes encoding the sterol biosynthetic enzymes. In addition SREBP-2 controls expression of the LDL receptor gene.
Protease-mediated regulation of SREBP activation.
S Of Cholesterol Synthesis
- The first step is the synthesis of isopentenyl pyrophosphate and activated isoprene unit, and it acts as the chief building block of cholesterol.
- The second step is the condensation of the six molecules of isopentenyl pyrophosphate for the formation of squalene.
- The third step is where the squalene cyclizes in a significant reaction with a tetracyclic product that converts into cholesterol.
Read Also: Ldl Cholesterol Range For Female
Stages Of Cholesterol Synthesis
There are three stages of cholesterol synthesis: oxidation, cyclization, and the loss of three methyl groups resulting in the conversion of squalene to cholesterol. The isoprene units or the isoprenoids are typically a class of large hydrophobic or nonpolar compounds related to this process and are constructed biosynthetically from the five-carbon units.
Cholesterol Esterification And Transport
Cholesterol is an amphipathic molecule , and in its native state it can freely diffuse through membranes. In order to be stored in cells, cholesterol must be modified by increasing its hydrophobicity. Cholesterol ester production in the liver is catalyzed by acyl-CoAcholesterol acyl transferase . ACAT catalyzes the transfer of a fatty acid from coenzyme A to the hydroxyl group on carbon 3 of cholesterol. Regardless of whether the additional group is an acyl chain or phosphatidylcholine, the resulting cholesterol esters are more hydrophobic than free cholesterol. The liver packages some of the esterified cholesterol into the hollow core of lipoproteins, primarily VLDL. VLDL is secreted from the hepatocyte into the blood and transports the cholesterol esters to the tissues that require greater amounts of cholesterol than they can synthesize de novo. These tissues then use the cholesterol for the synthesis of membranes, the formation of steroid hormones, and the biosynthesis of vitamin D.
Recommended Reading: How Long Should You Wait Between Cholesterol Tests
Introduction To Cholesterol Metabolism
Cholesterol is an extremely important biological molecule that has roles in membrane structure as well as being a precursor for the synthesis of the steroid hormones, the bile acids, and vitamin D. Both dietary cholesterol, and that synthesized de novo, are transported through the circulation in lipoprotein particles. The same is true of cholesteryl esters, the form in which cholesterol is stored in cells. Due to its important role in membrane function, all cells express the enzymes of cholesterol biosynthesis.
The synthesis and utilization of cholesterol must be tightly regulated in order to prevent over-accumulation and abnormal deposition within the body. Of particular clinical importance is the abnormal deposition of cholesterol and cholesterol-rich lipoproteins in the coronary arteries. Such deposition, eventually leading to atherosclerosis, is the leading contributory factor in diseases of the coronary arteries.
Structure of cholesterol
Proteolytic Degradation Of Hmg
The amount of HMG-CoA reductase can also be influenced by proteolytic degradation. The membrane domains of HMG-CoA reductase contain sterol-sensing regions, which are similar to those in SCAP. As levels of cholesterol increase in the cell, this causes a change in the oligomerization state of the membrane domain of HMG-CoA reductase, rendering the enzyme more susceptible to proteolysis. This, in turn, decreases the activity of the enzyme.
You May Like: Are Shellfish High In Cholesterol
Metabolic Engineering Strategies For Heterologous Production Of Sterols And Steroids In Yeast
To enhance the heterologous production of sterols and steroids in yeast, various metabolic engineering strategies have been developed based on the endogenous regulation mechanisms. Typical strategies include regulating the ergosterol synthesis pathway via enhancing the pathway flux by restriction of the competing branches, strengthening precursor supply, overexpression of rate-limiting enzymes, and/or reconstruction of cofactor balance and regulating sterol homeostasis via deletion or overexpression of the transcriptional factors, regulation of the accumulation of free sterols, and/or regulation of lipid metabolism .
Several Fates Of Cholesterol
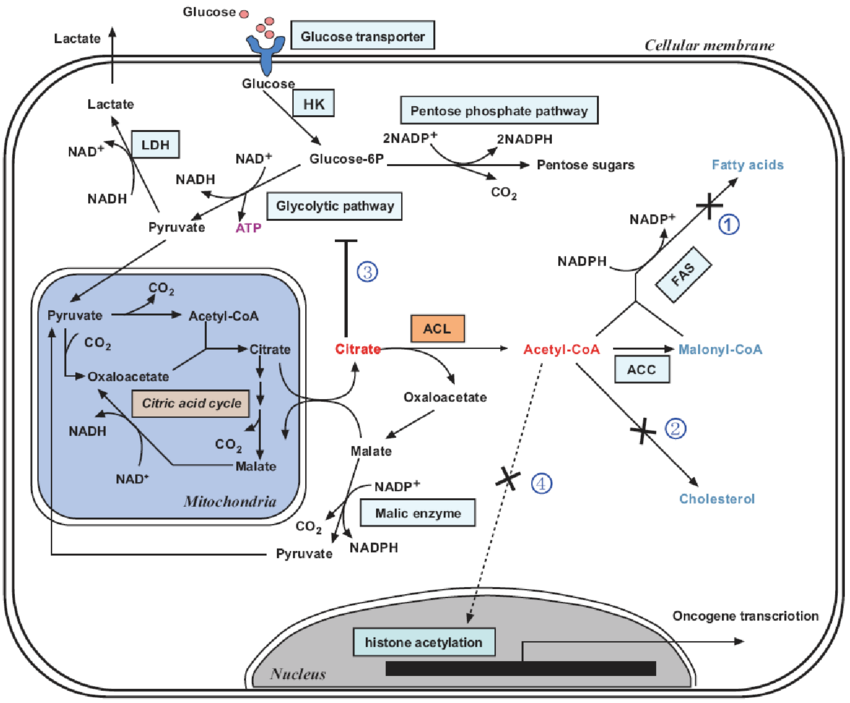
Almost all mammalian cells are capable of producing cholesterol. Most of the biosynthesis of cholesterol occurs within liver cells, although the gut, the adrenal cortex, and the gonads also produce significant quantities of the sterol. A small portion of hepatic cholesterol is used for the synthesis of hepatic membranes, but the bulk of synthesized cholesterol is secreted from the hepatocyte as one of three compounds: cholesterol esters, biliary cholesterol , or bile acids.
Recommended Reading: Is Tuna Good To Lower Cholesterol
Transcription Factor And Cholesterol De Novo Synthesis Enzymes
Several steps are required to convert acetyl-CoA to cholesterol, which then is involved in numerous biological roles. These steps involve cholesterol synthase , acyl coenzyme A, cholesterol acyltransferases , and ATP-binding cassette transporter A-1 . In a situation of decreasing cholesterol availability, inhibiting these enzymes could influence cancer cell growth. Interestingly, many inhibitors of these enzymes have effects on cancer treatment .1). SREBPs, which were reported the most transcription factors regulate cholesterol de novo synthesis. Also, KLF1422, ChREBP23,24, LXR25 and LRH-126 have very important roles in cholesterol metabolism. Due to the limitation of words, we just reviewed the role SREBP played on it.
Cholesterol biosynthesis pathway in cancer cells. Inhibitors of HMGCR, statins could exert anti-cancer effects through AKT, p53, BMP, ROS. And OSC through PI3K promoted cancer growth. To sum up, HMGCR, SQLE, OSC, ACAT1, SOAT and ABCA1 are the contributing factors in cancers. Statins, ACAT2 and ABCA1 are inhibitors in cancers. SREBP, sterol regulatory element binding protein ACAT1/2, acetyl-CoA acetyltransferase 1/2 SOAT, sterol-o-acyltransferase HMGCR, hydroxy-3-methylglutaryl-coenzyme a reductase SQLE, squalene epoxidase OSC, oxidosqualene cyclase ABCA1, ATP-binding cassette transporter A-1 PI3K, phosphatidylinositol 3-kinase AKT, protein kinase B ROS, reactive oxygen species BMP, bone morphogenetic protein.