Analysis Using The Gas Chromatograph14
How Do You Analyze Fatty Acids
Fatty acids are commonly analyzed by gas chromatography after conversion to fatty acid methyl esters which are more easily separated and quantified than either triglycerides or free fatty acids. In most methods the fat is saponified, which liberates the fatty acids from triglycerides, phospholipids, etc.producing free acids. The free acids are trans-esterified to form fatty acid methyl esters. Matrices that are not pure fats and oils require an extraction step to liberate the fat for analysis. Most solid samples are hydrolyzed by strong acid and/or akali, then extracted with organic solvents. In order to accurately quantify the fatty acid content of the sample as a weight percentage of sample, a synthetic fatty acid is added to the sample prior to extraction as an internal standard. The use of the internal standard compensates for variability in both the preparation and analysis of the sample.
The fatty acid methyl esters are then separated on the GC and quantified using a flame ionization detector . Separations are performed with wax type capillary columns when only basic chain length and saturation are needed. In order to quantify cis versus trans isomerization specialized, highly-polar capillary columns are used. The FID burns the FAMEs producing ions generating an electrical current which is measured and plotted as the response in the chromatogram.
Analysis Of Vegetable Oils Using Gass Chromatography
Typically, GC is suitable for the analysis of organic, non-ionic compounda that are vapourisable at 400°C or less. Therefore, for the analysis of lipids in edible oils, normally derivatisation of the lipids into their more volatile derivatives is performed prior to GC analysis. However, GC can also be used to analyse triglycerides or other components of oild that can be vapourised at 400°C or less. Here we describe the analyses of a few vegetable oils by capillary gas chromatograph .
- Content Type:
- Gas Chromatography
- Keywords:
- Fats and oils, GC 2010, Soy bean oil, canola oil, sunflower oil, olive oil, Food and Beverages, GC 2010, AOC-20i auto injector
- Language:
Most of the documents on the LITERATURE is available in PDF format. You will need Adobe Acrobat Reader to open and read PDF documents. If you do not already have Acrobat Reader, you can download it free at the Adobe’s Website. Click the GET ADOBE READER icon on the left to download a free copy of Adobe Acrobat Reader.
For Research Use Only. Not for use in diagnostic procedures.
This page may contain references to products that are not available in your country. Please contact us to check the availability of these products in your country.
You May Like: What To Do If You Have High Cholesterol
Separation Of Lipid Classes By Solid Phase Extraction 10
Rapid Analysis Procedures For Triglycerides And Fatty Acids As Pentyl And Phenethyl Esters For The Detection Of Butter Adulteration Using Chromatographic Techniques
Monica Gallo
1Department of Chemical Sciences, University of Naples Federico II, Via Cintia 4, 80126 Naples, Italy
2Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Via Pansini 5, 80131 Naples, Italy
Abstract
This paper presents the development of three methods for quality control, fraud detection, and authentication of butter fat and other oils/fats using chromatographic techniques, with one method for triglycerides and two methods for fatty acids . The procedure for the analysis of triglycerides requires only dissolution of the sample in -hexane and gas chromatography analysis using a capillary column. The second method is based on the transesterification of triglycerides as pentyl esters in a single-step reaction using sodium pentanoate in pentanol. The reaction proceeds at room temperature and is similar to the potassium hydroxide-catalysed transesterification of triglycerides with methanol and even more similar to the sodium methoxide method and sodium butanoate method. The advantage of using pentyl esters includes reducing the volatility of short-chain FAs, and substantial recoveries were obtained compared with methyl ester analysis. The third method involves the transesterification of triglycerides in fat through reaction with 2-phenylethanol in a single step 2-phenylethanol possesses a chromophore, and the phenethyl esters formed are analysed by high-performance liquid chromatography with UV detection.
1. Introduction
Read Also: Does Drinking Water Lower Cholesterol
Gas Liquid Chromatographic Analysis Of Fa
For GLC of FA, an aliquot of LD equivalent to 1.52 g protein is extracted as described above. FA are analyzed by GLC after hydrolysis and conversion to methyl esters. Therefore, 1 mL of a 2.5% H2SO4 in methanol solution is added to lipid extracts in a glass Pyrex tube which is carefully closed with the cap. After heating the samples in a heating chamber at 80 °C for 90 min and cooling them down to RT, 1 mL H2O and 3 mL light petroleum are added. FA methyl esters formed are extracted by shaking the tubes on the Vibrax for 30 min. After centrifugation at 1500 rpm for 5 min at RT, the organic phase is transferred into a new Pyrex tube and the extraction procedure is repeated with another 3 mL of light petroleum. The collected organic phases are dried under a stream of nitrogen, samples are dissolved in 100 L light petroleum, and transferred into GLC vials. FA methyl esters are separated by GLC using a Hewlett-Packard 6890 gas chromatograph equipped with an HP-INNOWax capillary column . Aliquots of 1 L are injected in split mode with helium as a carrier gas at a flow rate of 1.4 mL linear velocity 30 cm/s. The following program is used: 160 °C with 7.5 °C/min to 250 °C . Finally, FA are identified by comparison to commercially available FA methyl ester standards .
Jeff G. McDonald, … H. Alex Brown, in, 2016
Triglycerides In Oils Analysis By The 5975
Introduction Analytical conditions summaryGC-MS with Cold EI System:Samples:Injection:Column: He column flow rate:Oven: Cold EI Source:Transfer line temperatures:Mass spectral range and scan speed:Summary of the results
Also Check: What Do Cholesterol Numbers Mean
Fatty Acid Results And Reports
Most fatty acids are bound to glycerol as triglycerides and may exist in various other molecules , which may contain one or more different fatty acids. However, fatty acids are typically quantified as individual fatty acid methyl esters. This leads to various methods for reporting the final quantified results. Traditionally fatty acid results have been reported as % of the total area for all peaks. This method works well enough for refined vegetable oils where the fatty acid content is > 95% of the total mass. The method works less well for oils that contain significant amounts of non-triglyceride constituents. It is also prone to greater variability between laboratories depending on the exact conditions of the analysis. Eurofins Food Testing Laboratories will report results on this basis only when specifically requested.
Saturated fat and trans fat for nutrition labels are required to be listed as fatty acids per NLEA regulations. Total fat is required to be reported as triglyceride equivalents.
Preparation Of A Total Lipid Extract9
Read Also: What Is The Daily Intake Of Cholesterol
Pure Component Selective Reactivity
Figures and illustrate the effect of several reaction parameters on the overall conversion of FFAs and TAGs to FAMEs. The most important parameters governing the conversion are temperature and time. Figure A shows that conversion of FFA is highly dependent on temperature, with reactions conducted at as low as 50°C achieving complete conversion of the reactants into their corresponding FAMEs, whereas the same reaction conducted at 40°C yielded incomplete conversion. The sensitivity of the FFA esterification reaction to temperature is significant, because a 10°C increase in temperature raised the conversion from 70% to nearly 100% with a reaction time constant at 120 min and HClFFA ratio constant at 2:1. It appears that reactions above a temperature of 50°C did not improve the conversion of FFAs, and so it was determined that a reasonably mild temperature could be used to convert FFAs to FAMEs. The role of temperature in TAG conversion is shown in Figure A. It was determined that temperature had a significant impact on the completeness of TAG conversion, with a temperature of 80°C yielding over 90% conversion to ester, while a temperature of 40°C limited the conversion to approximately 10%. Clearly, when trying to minimize TAG conversion, a lower reaction temperature should be used.
Chromatograms illustrating the selective esterification procedure on a mixture of FFA and TAG: starting material reaction products after selective esterification procedure .
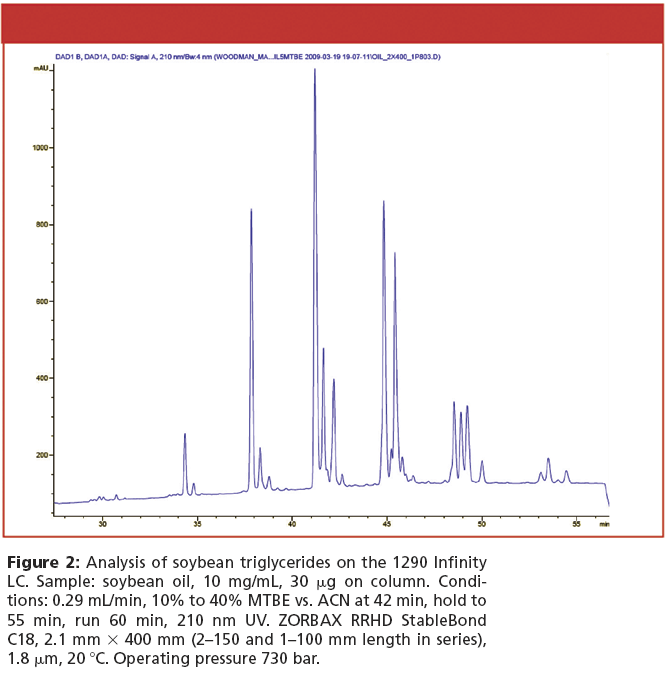
Accurate Reliable Gc Analysis Of Triglycerides In Edible Oils
- High temperature stability ensures consistent results and longer column lifetimes.
- Observe even underivatized mono- and diglycerides.
Edible oils, especially olive oil, are big business. Its why honest producers strive for a quality product, and its why others cheat consumers by selling adulterated goods that have been blended with or completely replaced by cheaper, lower-quality oils. To protect the industry, food scientists require analytical solutions that dependably determine quality and authenticity.
For decades, GC columns with 65% phenyl-substituted polysiloxane stationary phases have been used to analyze triglycerides in edible oils. But, quality and consistency vary significantly because evenly coating these phases inside column tubing is very difficult to do. As a result, 65-type columns can exhibit high bleed and low inertness. Bleed interferes with accurate identification and quantitation, and over time it leads to shifts in retention time, loss of resolution, and poor peak shape due to increasing column activity. The relatively high temperatures used in most triglyceride methods only exacerbate these problems.
Don’t Miss: Is Coconut Milk Good For Cholesterol
Preparation Of Fame From Ce10
Selective Reactivity Of Sesame Oil Components
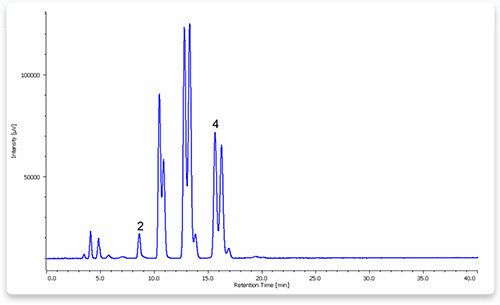
To demonstrate method effectiveness on a complex biological oil, a well-characterized sesame oil standard was reacted under the optimized selective esterification conditions . The oil was also subjected to a more rigorous esterification reaction conducted at 80°C for over 100 min, which was done to convert the entire FFA and TAG component to FAMEs. Additional experiments were performed with this sesame oil that was spiked with 0.50% FFA as oleic acid. The composition of the selective esterification reaction product was determined by GCFID and compared to that of the fully esterified reaction product . The results of these spike experiments are shown in Table . The accuracy of the selective esterification method was established by confirming that the total FFA content of the unspiked oil was lower than the spiked oil by the amount of oleic acid spike. Moreover, by tracking just the concentration of oleic acid itself, the method was able to effectively recover the 0.5% that was spiked into the oil.
Recommended Reading: What Makes Your Cholesterol High
Removal Of Free Cholesterol Contamination From Ce Fame14
Consistent Performanceevery Column Every Time
Figure 1 demonstrates the consistent performance of Rxi-65TG columns. Tightly controlled manufacturing and rigorous QC testing ensure that every new Rxi-65TG column will perform as well as the last. We even monitor the symmetry of undecanol, an active probe that is an excellent indicator of column inertness, to ensure that Rxi-65TG columns are inert enough to observe underivatized mono- and diglycerides .
Figure 1: Rxi-65TG columns provide dependable performance column to column and lot to lot.
Also Check: How Much Saturated Fat Per Day High Cholesterol
Dependable Results Even Under Extreme Conditions
The truest test of thermal stability is how a column performs after long-term exposure to stressful conditions. Columns used for triglycerides analysis are routinely cycled above 360 °C for short periods, so we created a more rigorous test that compared the columns after a total high temperature exposure time of 48 hours. In the experiment detailed below, the final oven temperature was held to 360 °C for all columns because it was the maximum temperature of the competitor columns . As shown in Figure 3, the Rxi-65TG column has the lowest bleed throughout the experiment, which means you can reliably separate and quantify triglycerideseven after days of cumulative high-temperature exposurewithout interference from bleed. In addition, only the Rxi-65TG column was inert enough that both mono- and diglycerides could be observed.
Figure 3: Only thermally stable Rxi-65TG columns provide consistent, low-bleed performance even after 48 hours at 360 °C.
Rxi-65TG Column