Cholesterol Molecules Avoid Being Located In Adjacent Positions
We start by analyzing the pair correlation functions, g, for DPSC/Chol membranes at different Chol concentrations. The variable r stands for the radial distance from the center of mass of a cholesterol molecule and g measures density variation with respect to the average density as a function of distance. The results are shown in Figure 2. For a Chol-DSPC pair , g has its main peak at a distance of 0.5 nm. As this is also the first peak, it corresponds to the first coordination shell. In contrast, the main peak for Chol-Chol pair correlations is located at 1.0 nm. This is the second peak and hence corresponds to the second coordination shell. The physical interpretation of this observation is that cholesterols avoid being located in adjacent positions , . This behavior is common in all systems with moderate cholesterol concentrations. Only at high cholesterol concentrations does the occurrence of close contacts become relevant since then the membrane is crowded with cholesterol molecules.
Why Is A Cholesterol Molecule Nonpolar
4.8/5moleculecholesterolnon-polar moleculemolecule
Cholesterol has a small, water-soluble polar region that dissolves in water, but nearly the entire cholesterol molecule is non-polar, which will NOT dissolve in water like oil. This makes cholesterol an example of an amphipathic molecule part water-soluble, part water-insoluble.
Additionally, why are lipids non polar? The carbon to carbon and carbon to hydrogen bonds found in lipids are considered nonpolar. This means the electrons in the bond are shared relatively equally between the atoms. These slight charges on the atoms in the water molecule, called dipoles, result in water being referred to as a polar molecule.
One may also ask, which part of a cholesterol molecule is polar?
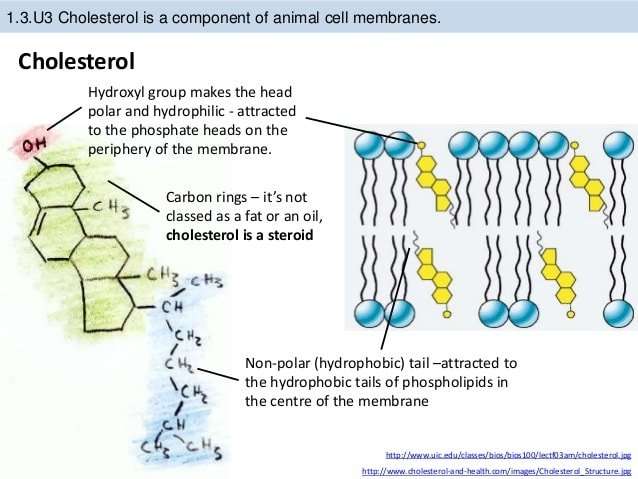
The ring-like structures are fairly rigid, but there is also a hydrocarbon tail, which is somewhat flexible. The entire structure is somewhat reminiscent of a fancy kite with a tail. Cholesterol is very non-polar, except for the hydroxyl group attached to the first ring.
Why do polar and nonpolar molecules repel?
When things are different at each end, we call them polar. Some molecules have positive and negative ends too, and when they do, we call them polar. If they don’t, we call them non-polar. Things that are polar can attract and repel each other .
Amino Acid Substitutions In Domain 3 Modify The Cholesterol
As described above, modifications to the PFO D4 alter the cholesterol threshold of the protein. When the F318A change was introduced in the distal D3 of the protein to generate the FPFO derivative , no variations in the cholesterol threshold were observed. The substitution of a second aromatic residue in D3 unexpectedly reduced the cholesterol threshold of the protein to levels like those observed for the wild type nPFO . Given the long distance that separates Y181 from the D4 tip, it is clear that modifications outside the conserved loops can alter the cholesterol binding properties of PFO derivatives, presumably through the coupling between D3 and D4,. We have therefore generated a parental non-lytic PFO derivative , with the same cholesterol threshold as the wild type protein .
Figure 3
Read Also: How Does Cholesterol Leave The Body
How Cholesterol Interacts With Membrane Proteins: An Exploration Of Cholesterol
- 1EA-4674, Interactions Moléculaires et Systèmes Membranaires, Aix-Marseille Université, Marseille, France
- 2Laboratory of Molecular Neurobiology, Faculty of Medical Sciences, Biomedical Research Institute UCACONICET, Catholic University of Argentina, Buenos Aires, Argentina
A Perspective On Size
An angstrom is one ten-millionth of a millimeter, or 1×1010 meters. The illustration below gives an idea of the relative scale of some of the biological structures discussed above.
The distance between two carbon atoms in a fatty acid chain is a little over one angstrom. A glucose molecule is about 9 angstroms. Bacteria are tens of thousands of angstroms. And as a rough estimate, a typical human cell might be approximately 1/100th of a millimeter which is about 1/10th the width of a human hair. For an intriguing perspective on the size of things from the smallest to the largest objects in the universe, take a look at http://htwins.net/scale2/.
Despite their microscopic size, cells have a lot going on all the time. Diagrams and photomicrographs depict cells as rigid, static sacs that are frozen in time, but if we could somehow take a trip inside a cell, we would be staggered by the beauty, complexity, and incredible activity. You can get at least a glimpse of the inner life of a cell by watching the Harvard University animation, The Inner Life of a Cell , showing leukocyte activation in inflammation. Some of the terms used in the video will be foreign to you, but the video provides a magnificent sense of the inner works of cells, and it shows cells to be dynamic structures in which many processes are taking place continually.
Read Also: How Much Cholesterol In A Baked Potato
Recommended Reading: Does Exercise Affect Cholesterol Test
General Behaviour Of Lipids In Water
From a biological standpoint an essential property of lipid molecules is their ability to form aqueous phases, possessing long-range order combined with disorder at molecular distances. A variety of different phases exist for a particular lipid , and a small change in solution conditions is sufficient to cause a transformation from one form or structure to another.
It is natural to classify lipids as polar or non-polar according to their interaction with water. Non-polar lipids, for example triglyceride oils, do not form aqueous phases, whereas polar lipids do. Except for cholesterol, membrane-forming lipids form aqueous phases and have polar head groups. Within membranes there are also trace amounts of lipids in membranes that do not interact with water, for example diacylglycerols. The structural formulae of two common membrane lipids, phosphatidylcholine and phosphatidylethanolamine are shown above.
The physical properties of polar lipids, such as phospholipids, glycolipids or monoglycerides, are directly related to their association, behaviour.
Liquid crystalline phases form more commonly in the presence of water . Above a critical hydrocarbon chain melting temperature, water penetrates the polar region, and a lamellar lipid-water structure is formed with water layers alternating with lipid bilayers .
William W. Christie, Xianlin Han, in, 2012
Are Lipids Polar Or Non
Polar molecules are those where molecules do not share electrons equally between them within a covalent bond. It leads to the formation of a net electrical charge across the molecule. Whereas, non-polar molecules are those where molecules share electrons between them equally forming a strong covalent bond. It leads to the formation of no net electrical charge across the molecule. This formation of a net electrical charge makes molecules more receptive to any change in the nearby environment, as every atom wants to achieve the most stable condition.
So, are lipids polar or nonpolar? The lipids are non-polar in nature due to the uniform distribution of charge among the carbon and hydrogen atoms present in the molecule. As there is no net partial charge on the overall lipid molecule, it is non-polar in nature.
Recommended Reading: What Foods Are Good To Lower Your Cholesterol
Do Polar Molecules Elute First
In normal-phase chromatography, the least polar compounds elute first and the most polar compounds elute last. Retention decreases as the amount of polar solvent in the mobile phase increases. In reversed phase chromatography, the most polar compounds elute first with the most nonpolar compounds eluting last.
Why Are Lipids Non Polar
lipidsnonpolarpolar
. Keeping this in consideration, are lipids non polar?
Explanation: Lipids are a group of molecules that includes fats, fatty acids, sterols, and phospholipids. Although they have polar functional groups at one end, the hydrocarbon parts of the molecules are so large that the molecules are hydrophobic. Lipids are effectively nonpolar and insoluble in water.
Likewise, why is cholesterol mostly nonpolar? As this molecule is composed of mainly hydrogen and carbon atoms , cholesterol is considered to be a non-polar molecule even though there is a small polar hydroxyl group. The non-polarity of the molecule is evident due to the fact that it cannot dissolve in water.
Regarding this, why are lipids insoluble in polar solvents?
Thus, polar molecules are considered to be hydrophilic and lipophobic , due to the ability of highly polar bonds being soluble in polar solvents, and insoluble in nonpolar solvents , and are soluble in nonpolar molecules.
Are lipids polar or nonpolar quizlet?
Lipids are NON polar because we don’t want them mixing with water. THey make up cell membranes and we wouldn’t want them to mix with the watery cytoplasm.
Don’t Miss: What Happens If You Eat Too Much Cholesterol
B Lipids: Membranes And Fats
A note from Dr. Haas: Lipids are molecules that are mostly nonpolar, but have some polar character. These molecules serve important biological functions, such as providing the principle component of membranes and serving as energy storage . The structures of a triglyceride and a phospholipid are shown above. Triglycerides are the things we commonly refer to as fats and oils. Phospholipids are similar to triglycerides with one important difference.
A skeletal structure of a phospholipid and a triglyceride are shown above. Notice the similarities and differences between the two structures. The phospholipid is similar to the triglyceride in that it contains fatty acid tails attached to a glycerol backbone. However, the phospholipid contains a organic phosphate zwiterion instead of a third fatty acid tail.
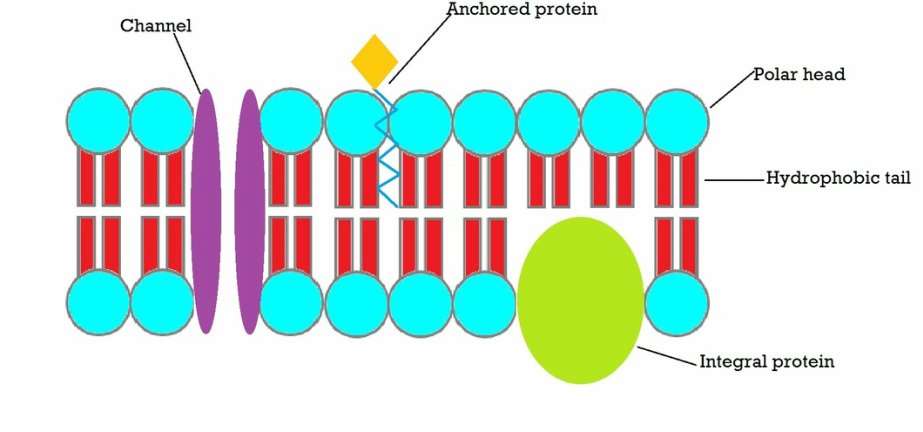
Triglycerides are completely insoluble in water. However, due to the ionic organic phosphate group, phospholipids demonstrate properties because the ionic group is attracted to water. Phospholipids have both a polar, hydrophilic end, and a nonpolar, hydrophobic end. Phospholipids are partially soluble in water, meaning that part of the molecule is attracted to water, and part of it is not. Phospholipids form important structures in water when the polar end faces water and the nonpolar end faces away from water. Below is a cartoon version of the phospholipid bilayer in 2D.
How Does Electronegativity Determine The Non
The electronegativity of an atom is a tendency to attract a shared pair of electrons. It depends upon the atomic number of the element and the distance between the nucleus and the valence electrons.
Most of all chemical reactions take place when molecules having lower bond energies collide with one another.
As they have low bond energy, it doesnt take much effort to separate them from one another.
However, they make molecules of much stable condition when they reform into new elements. Moreover, these much stable elements have great bond energy which holds molecules together in a much stronger manner.
The shared electrons within a covalent bond are pulled by protons, at the center of the two atoms.
As non-polar molecules are much stable, their electronegativity value will be much lower than the polar molecules.
So, if the electronegativity difference within the atoms in a covalent bond is greater than 0.4, the molecule is polar.
Whereas, if the electronegativity difference within the atoms in a covalent bond is lesser than 0.4, the molecule is non-polar in nature. Lipids being a non-polar molecule has an electronegativity value lesser than 0.4.
Recommended Reading: What Causes High Cholesterol In Vegetarians
Phospholipids And Biological Membranes
Figure 2. This illustration shows a phospholipid with two different fatty acids, one saturated and one unsaturated, bonded to the glycerol molecule. The unsaturated fatty acid has a slight kink in its structure due to the double bond.
Triglycerides are classified as simple lipids because they are formed from just two types of compounds: glycerol and fatty acids. In contrast, complex lipids contain at least one additional component, for example, a phosphate group or a carbohydrate moiety . Figure 2 depicts a typical phospholipid composed of two fatty acids linked to glycerol . The two fatty acid carbon chains may be both saturated, both unsaturated, or one of each. Instead of another fatty acid molecule , the third binding position on the glycerol molecule is occupied by a modified phosphate group.
Figure 3. Phospholipids tend to arrange themselves in aqueous solution forming liposomes, micelles, or lipid bilayer sheets.
Some Other Related Questions
What are valence electrons?
Valence electrons are those electrons that are present in the outermost shell of an atom that participates in the formation of a chemical bond.
A bond formation needs to contribute at least one valence electron from both atoms. These are the electrons that make weak hydrogen bonds and shared strong covalent bonds in the polar and non-polar molecules.
The structural difference between saturated and unsaturated fatty acids.
The basic structural difference between saturated and unsaturated fatty acids is that the saturated ones are straight chains.
Whereas, the unsaturated fatty acids are bent because of the absence of two hydrogen atoms and the existence of a double bond between carbon atoms. The unsaturated fatty acids are more stable than the saturated ones.
Also Check: Is Pizza High In Cholesterol
You May Like: Is Keto Diet Ok For High Cholesterol
Metabolism Recycling And Excretion
Cholesterol is susceptible to oxidation and easily forms oxygenated derivatives called oxysterols. Three different mechanisms can form these: autoxidation, secondary oxidation to lipid peroxidation, and cholesterol-metabolizing enzyme oxidation. A great interest in oxysterols arose when they were shown to exert inhibitory actions on cholesterol biosynthesis. This finding became known as the “oxysterol hypothesis”. Additional roles for oxysterols in human physiology include their participation in bile acid biosynthesis, function as transport forms of cholesterol, and regulation of gene transcription.
In biochemical experiments radiolabelled forms of cholesterol, such as tritiated-cholesterol are used. These derivatives undergo degradation upon storage and it is essential to purify cholesterol prior to use. Cholesterol can be purified using small Sephadex LH-20 columns.
Although cholesterol is a steroid generally associated with mammals, the human pathogen Mycobacterium tuberculosis is able to completely degrade this molecule and contains a large number of genes that are regulated by its presence. Many of these cholesterol-regulated genes are homologues of fatty acid-oxidation genes, but have evolved in such a way as to bind large steroid substrates like cholesterol.
Medical Guidelines And Recommendations
In 2016, the United States Department of Agriculture Dietary Guidelines Advisory Committee recommended that Americans eat as little dietary cholesterol as possible, because most foods that are rich in cholesterol are also high in saturated fat and thereby may increase the risk of cardiovascular disease. Previously, the Dietary Guidelines for Americans recommended that dietary cholesterol be no more than 300 mg per day. The DGAC dropped this recommendation because evidence showed no appreciable relationship between dietary and serum cholesterol, consistent with the 2013 report by the American Heart Association and the American College of Cardiology. Although there is a link between cholesterol and atherosclerosis, a 2014 review concluded there is insufficient evidence to support the recommendation of high consumption of polyunsaturated fatty acids and low consumption of total saturated fats for cardiovascular health.
Some supplemental guidelines have recommended doses of phytosterols in the 1.63.0 grams per day range . A recent meta-analysis demonstrating a 12% reduction in LDL-cholesterol at a mean dose of 2.1 grams per day. However, the benefits of a diet supplemented with phytosterols have also been questioned.
| > 6.2 | High risk |
Don’t Miss: How Much Will Statins Lower My Cholesterol
Is Cholesterol Polar Or Non
Cholesterol is an organic molecule that belongs to the steroid family. It can be found in body tissues such as the liver, brain, spinal cord as well as in the blood plasma.
This waxy substance is extremely important in order for the body to carry out several functions such as producing steroid hormones , vitamin D, and other compounds from which the body synthesizes bile acids.
Moreover, cholesterol plays a huge role in brain synapses and in the immune system.
In terms of polarity, determining whether a molecule is polar or non-polar is important in order to find out whether said molecule is attracted or repelled to water.
Molecules with high polarity are hydrophilic also called water-loving, whereas molecules with low polarity are hydrophobic, also known as water-fearing.
Now here comes the big question, is cholesterol polar or non-polar? Keep on reading to find it out.
Analysis Of Crystallographic Structures Containing Cholesterol Sulfate
The analysis of the few available crystal structures of cholesterol may not be enough to define common features of cholesterol sites in soluble proteins. Thus, we also studied cholesterol sulfate. This is a cholesterol derivative in which the 3-hydroxyl is substituted by a sulfate group. Although this substitution diminishes the overall hydrophobicity of the molecule, the remaining structural features are identical to those of cholesterol. From a biological standpoint, cholesterol sulfate has been extensively recognized as one of the most important sulfonated steroids. Higher levels of cholesterol sulfate were found in the plasma of patients with liver cirrhosis and hypercholesterolemia while atherosclerosis has been linked to cholesterol sulfate deficiency . Under normal physiology, cholesterol sulfate plays a critical role in platelet adhesion and keratinocyte differentiation. At the molecular level, this steroid regulates the activity of serine proteases and, in a rather selective manner, of protein kinase C isoforms. Several PDB entries describe cholesterol sulfate-protein complexes. We analyzed three complexes found in the PDB database, their topology being depicted in . Averaged volume of the site was estimated at 2,745 ± 256 Å3. The key features of cholesterol sulfate binding sites are summarized in .
Also Check: How Do Cheerios Lower Cholesterol
Recommended Reading: Is Eating Peanut Butter Bad For Your Cholesterol
Quantification Of Individual Lipid Species By Multi
After separation of different lipid classes in the ESI ion source and identification of individual species by multidimensional MS , quantification of the identified individual species of a class of interest is performed in a two-step procedure as outlined previously . This procedure can now be conducted automatically . Briefly, this methodology first determines whether there exist overlapping ion peaks with the peaks of species of other lipid classes or of low abundance. The method performs the one-step quantification procedure for the case of no overlapping peaks and no low abundance peaks and performs the two-step quantification procedure for the other cases.
To perform the one-step quantification procedure, a list of the identified ions of the class along with the number of total carbon atoms of these ions, and the peak intensity of each individual ion present in the full MS scan are loaded onto a spreadsheet. Baseline correction and de-isotoping for the peaks in the list are performed. The baseline-corrected, de-isotoped peak intensities are then generated and used to quantify the content of each ion peak by ratiometric comparison with the selected internal standard of the class as follows:
where cu and ci are the contents of individual species in the list and the selected internal standard, respectively, while Iu and Ii were the peak intensities of the species and the selected internal standard, respectively, after 13C de-isotoping.