Difference In Cholesterol Distribution In Ptdss1/ And Npc1/ Cells As Assessed By Fluorescence Microscopy
To demonstrate the cholesterol transport defect in PTDSS1/ cells morphologically, we incubated cell monolayers with FCS, stained the cells with AF488-PFO*, and examined them with a fluorescence microscope . The images revealed intense staining of the PM in the PTDSS1/ cells but not in the NPC1/ cells . A different result was obtained when we stained the cells with filipin, a cell-permeable fluorophore that binds cholesterol . Filipin showed intense staining of lysosomes in NPC1/ cells but not in PTDSS1/ cells, confirming that the PTDSS1/ cells have no block in the release of cholesterol from lysosomes.
Difference in cholesterol distribution in NPC1/ and PTDSS1/ cells as assessed by fluorescence microscopy. PM cholesterol. On day 0, WT, NPC1/, and PTDSS1/ CHO-K1 cells were plated on 12-mm glass coverslips in medium C with 5% FCS. On day 2, cells were switched to cholesterol-depletion medium C. After a 12-h incubation, cells were switched to medium D containing CPN/MEV and supplemented with 10% FCS. After 6 h, cells were imaged using AF-488conjugated PFO* Total cellular cholesterol. Cells were set up and incubated as above and then imaged with filipin
Ptdss: A Second Enzyme That Synthesizes Ps
In addition to PTDSS1, animal cells can synthesize PS through the action of PTDSS2 . In contrast to PTDSS1, which exchanges serine for choline, thereby converting PC to PS, PTDSS2 exchanges serine for ethanolamine, thereby converting PE to PS. In contrast to the PTDSS1 gene, which was among the highest-scoring genes in our CRISPR screen , the PTDSS2 gene was among the lowest , suggesting that PTDSS2 is not essential for transport of LDL-derived cholesterol in human SV589 cells . To confirm these results, we used CRISPR-Cas 9 to inactivate the PTDSS2 gene in CHO-K1 cells and compared these cells with CHO-K1 cells lacking PTDSS1. In contrast to the PTDSS1-deficient cells, in cells lacking PTDSS2, LDL did not produce an increase in PM cholesterol, and the LDL-derived cholesterol was esterified normally . These results indicate that PTDSS1 is the only enzyme in human SV589 cells or CHO-K1 cells that can synthesize the PS required for transport of PM cholesterol to the ER.
Bile Acid Cycling Involves Multiple Transport Proteins
A variety of transport proteins enable the bile acid enterohepatic cycle. Secretion from the liver cell into the bile is driven by ABCC2, another ABC type transporter . Reuptake from the lumen of the gut is mediated by the apical sodium-coupled bile acid transporter . A similar transporter, the Na+-dependent taurocholate cotransporting polypeptide , mediates uptake from the blood back into the liver cell. At the basolateral membranes of both intestinal and liver cells, organic anion transport proteins , which have a fairly low degree of substrate specificity, participate in bile acid transport.
| 11.5.4 |
You May Like: Do Clams Have Cholesterol
What Is The Function Of The Endoplasmic Reticulum
The endoplasmic reticulum serves important functions particularly in the synthesis, folding, modification, and transport of proteins. Differences in certain physical and functional characteristics distinguish the two types of ER, known as rough ER and smooth ER . Ribosomes on RER, which give RER its rough appearance, specialize in the synthesis of proteins that possess a signal sequence that directs them specifically to the ER for processing. Proteins synthesized by the RER have specific final destinations, such as the cell membrane, cell exterior, or the ER itself. SER is involved in the synthesis of lipids, including cholesterol and phospholipids, which are used in the production of new cellular membrane. In cells of the liver, SER contributes to the detoxification of drugs and harmful chemicals. The sarcoplasmic reticulum is a specialized type of SER that regulates calcium ion concentration in the cytoplasm of striated muscle cells.
Adrenal Microsomal Subfractions From Other Species
To eliminate the possibility that the presence of translocon-associated proteins in adrenal smooth microsomes was confined to the guinea pig, we examined similarly prepared microsomes from adrenals of several species: rat, dog, cattle, rabbit, sheep, and pig. In most, as in the guinea pig adrenal, OST and Sec61 complex subunits as well as the molecular chaperones BiP, GRP94, and calnexin, were found to be in equal or greater concentration in smooth microsomes compared with rough microsomes . The sole exceptions to the equal or greater concentration of these proteins in the smooth microsomes were in the rat adrenal. In the rat adrenal, the OST components as well as BiP and GRP94 were in greater concentration in the rough microsomes. In all of the species examined, however, the SER markers CYP17 and 3HSD were in greater concentration in the smooth microsomes than in rough microsomes. In all cases, the ribosomal protein S3 was localized to the ribosome bearing fractions . This gives increased confidence that the broader distribution of translocon-associated proteins in the ER is a property of most adrenocortical cells and perhaps of steroid-secreting cells in general.
Read Also: Does Eating Shrimp Raise Cholesterol
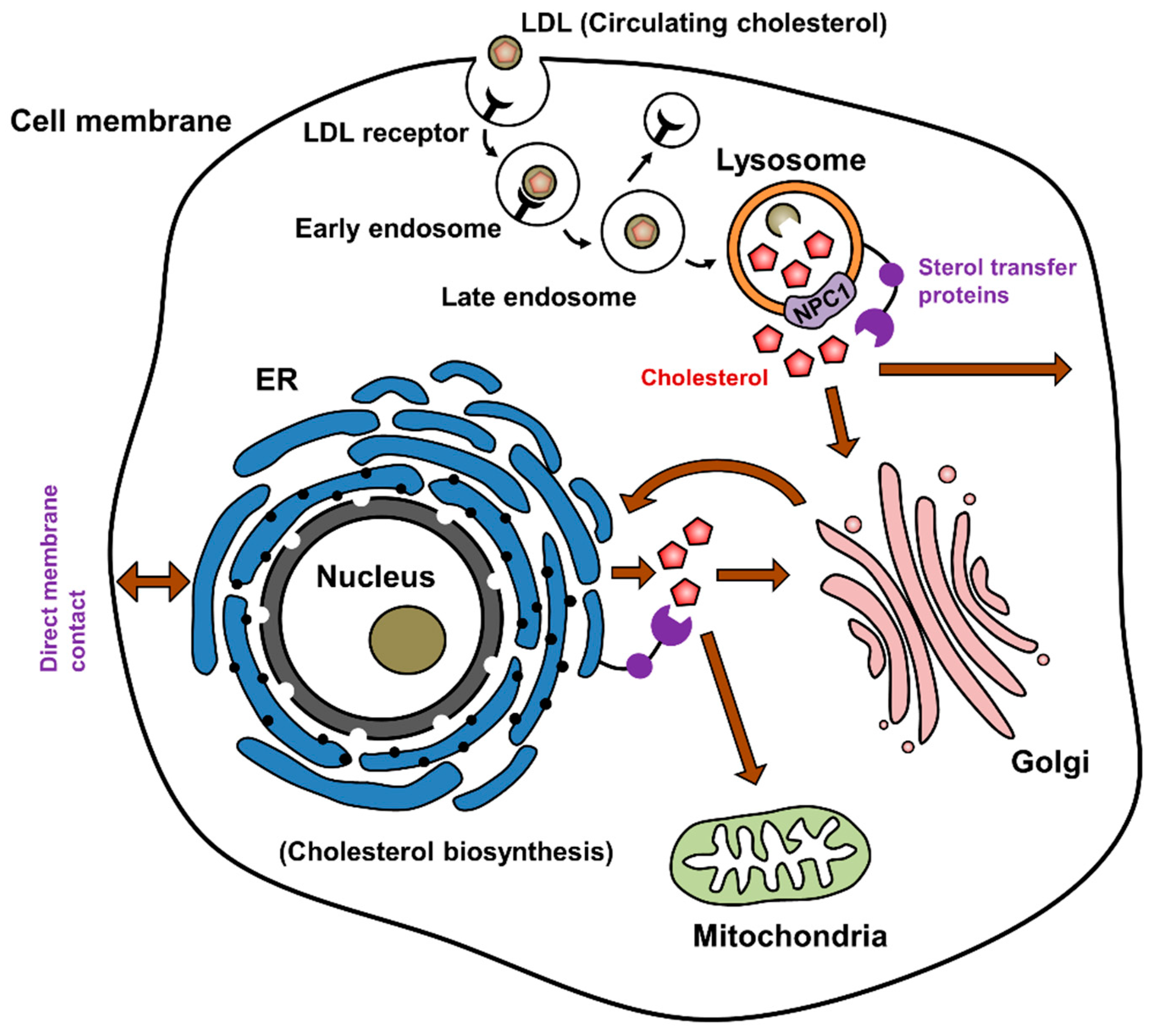
Npc1 Tethers Er Contact Sites With Late Endocytic Organelles
Using NPC1-deficient cells as models of defective cholesterol transport, we investigated the contribution of MCS to the egress of LDL-cholesterol from endocytic organelles using two complementary techniques: Live-cell fluorescence microscopy, enabling rapid screening of cellular organelles in their native environment, and electron microscopy where the higher resolution allows the contact itself to be visualised.
We first examined the association between late endosomes/lysosomes and the ER in control and Npc1/ Chinese hamster ovary cells by fluorescence microscopy. Using Sec61-GFP and LysoTracker to visualise ER and lysosomes respectively, we found significantly reduced association between the two organelles in Npc1/ compared to control cells as quantified using Pearsons correlation coefficient . A similar reduction in co-localization was achieved by acute pharmacological NPC1 inhibition using U18666A, which binds the sterol-sensing domain of NPC1 and induces cholesterol accumulation .
Fig. 1
Thus, we have revealed a dual functionality for STARD3, such that when ER contact with endocytic organelles is reduced, resulting in cholesterol accumulation, STARD3 instead promotes association between the cholesterol-laden lysosomes and mitochondria.
Cholesterol Provides Membranes With Special Physical Properties
It has been long known that cholesterol condenses and rigidifies lipid bilayers containing phospholipids with unsaturated fatty acyl chains. In contrast, it fluidizes bilayers of di-saturated phospholipids and sphingolipids, which in its absence would be in a solid gel phase. In the past several years, studies of three component lipid mixtures containing cholesterol have shown that cholesterol can profoundly affect the phase separation behavior of lipids. In particular, cholesterol can promote the formation of nanoscopic phase separations in mixtures of unsaturated phospholipids with saturated phospholipids and/or sphingolipids, which would otherwise consist of one fluid phase or a well separated macroscopic fluid and a gel phase . These small lipid domains in model membranes resemble the microdomains that can form in biological membranes, which are compositionally much more complex.
When eukaryotic cells obtained their internal membranes, they started to synthesize sterols and sphingolipids . This may have facilitated segregation of lipids into domains of different composition, which forms the basis for maintaining the 510-fold enrichment of sphingolipids and sterols in the plasma membrane as compared to the ER . Evidence for preferential inclusion of sterols and sphingolipids into anterograde transport vesicles derived from the trans-Golgi network has been reported recently as had their relative depletion from retrograde vesicles .
Recommended Reading: Are Pork Chops Heart-healthy
Demethylation Desaturation And Saturation Steps Convert Lanosterol To Cholesterol
Several successive modifications convert lanosterol to 7-dehydrocholesterol and then cholesterol. Like squalene epoxidase, several of the enzymes that catalyze these reactions also belong to the cytochrome P450 family. We will not consider them in detail however, several of these enzymes are inhibited by the drug triparanol, which was once used to treat hypercholesterolemia before being withdrawn due to toxicity.
11.2.7
Structures Of Abc Transporters In The Inward
Both ABCA5/8 and ABCA1 are members of the ATP-binding cassette or ABC family of transporters. These have a common structural organization. Several ABC transporters have been crystallized in the inward- and outward open conformations , and the two structures provide a glimpse of how they work.
ABC transporters often have rather broad substrate specificity and mediate the membrane translocation of many metabolites and xenobiotics. In addition to cholesterol and other membrane lipids, important examples are bile acids , conjugated bilirubin , drugs, and drug metabolites . Cancer cells often overexpress ABC transporters, which renders them resistant to multiple anticancer drugs.
11.4.6
You May Like: Beer Effect On Cholesterol
Which Type Of Organelle Is Primarily Involved In The Synthesis Of Oils Phospholipids And Steroids
4.5/5
| Question | Answer |
|---|---|
| Which type of organelle is primarily involved in the synthesis of oils, phospholipids, and steroids?a.contractile vacuole b.ribosome c.Smooth ER d.lysosome e.mitochondrion | c.Smooth ER |
Similarly one may ask, which organelle is responsible for synthesis of oils phospholipids and steroids?
smooth endoplasmic reticulum
Furthermore, what junction is involved and what organelle stores calcium? The endoplasmic reticulum, and its specialized muscle counterpart the sarcoplasmic reticulum, is the largest and most extensive of Ca2+ storage organelle in eukaryotic cells, often occupying in excess of 10% of the cell volume.
Correspondingly, which type of organelle or structure is primarily involved in the synthesis of proteins that may be exported from the cell?
Endoplasmic reticulum with attached ribosomes is called rough ER. It looks bumpy under a microscope. The attached ribosomes make proteins that will be used inside the cell and proteins made for export out of the cell.
Which organelle is the primary site of ATP synthesis in eukaryotic cells?
mitochondria
Squalene Cyclization Yields The First Sterol Intermediate
The reactions shown here are catalyzed by squalene epoxidase and lanosterol synthase. The rearrangement indicated by the dashed arrow is not a real reactionââ¬âwe just rotate a couple of single bonds to show how the pieces fall into place for the subsequent cyclization.
The oxygen is introduced by squalene epoxidase, a cytochrome P450 enzyme. Such enzymes use NADPH to reduce one of the two atoms of molecular oxygen, while retaining the other one in a highly reactive state, which they then use toward their specific purposes . Squalene synthase inserts its active oxygen into a C=C double bond of the substrate to form an epoxide. The subsequent cleavage of the epoxide by lanosterol synthase starts a cascade of reactions that goes from one end of the molecule to the other, closing all four rings of the sterol skeleton in the process. Note that a methyl group also changes its place on the sterol ring the reaction mechanism is quite intricate.
11.2.6
Don’t Miss: Does Shrimp Have Good Cholesterol
Analysis Of Ost Activity
The octanoyl tripeptide , N-octanoyl-Asn-Tyr-Thr-amide, which contains the acceptor sequence for N-glycosylation, was received from Dr. Felix Wieland . OTP was prepared and used as a glycosyl acceptor to assay oligosaccharyltransferase activity, as previously described . After incubation with microsomal protein for 1 h at 37 C in the presence and absence of the glucosidase inhibitor, castanospermine , the glycosylated tripeptide was bound to a conA Sepharose column and eluted with buffer containing methyl–d-mannopyranoside and Triton X-100. Activity in the eluate from the conA Sepharose column is expressed as counts per minute per microgram microsomal protein used in the assay.
Analysis of glycotripeptides by thin layer chromatography was performed as previously described . To further define the pattern of glycosylation, the eluted samples were subjected to enzymatic digestion with -mannosidase and Endo H . Aliquots containing approximately equal counts per minute from each sample were incubated with Jack bean -mannosidase or Endo H in the 1× buffer supplied with the -mannosidase, as described in the accompanying literature. The total incubation mixture was 59 l. After incubation at 37 C, overnight , the reaction mixture was spotted on TLC plates, without further processing and the components separated in the solvent system described above.
Cells Stably Expressing Gfp
pGFP-SCAP plasmid, encoding GFP fused to wild-type hamster SCAP under the control of the CMV promoter, was a gift from Dr. Peter Espenshade . pEGFP-ATF6- was a gift from Ron Prywes . CHO-K1, ZR-78.1C, ZR-82, and ZR-87 cells stably expressing GFP-SCAP were generated by transfection with pGFP-SCAP using polyethylenimine , followed by selection with G418. After selection with G418 GFP-SCAP-positive cells were sorted on a BD FACSAriaTM IIIu BL1 sorter . The plasmid containing full-length rat Pex2 cDNA was a gift from Masaki Ito .
You May Like: How Do You Test Cholesterol
Western Blotting And Immunoprecipitation
For Western blotting, cells were lysed in lysis buffer . Lysates were fractioned by SDSPAGE on 10% gels under reducing conditions and immunoblotted on nitrocellulose membranes. Following incubation with infrared-fluorophore-conjugated secondary antibodies , membranes were scanned in an Odyssey SA scanner . For IP, cells were lysed as above following crosslinking for 30min at RT in DSP crosslinking solution and blocking for 30min at RT in blocking solution . NPC1 immunoprecipitates were fractioned, immunoblotted and scanned as described for Western blotting. Supplementary Fig. shows uncropped blots for Fig. .
Intracellular And Plasma Membrane Events In Cholesterol Transport And Homeostasis
Alexander D. Dergunov
1National Research Centre for Preventive Medicine, 10 Petroverigsky Street, 101990 Moscow, Russia
Abstract
1. Introduction
The goal of this review is to describe the complex processes of cholesterol metabolism and cholesterol traffic inside the cell and the effect of these processes on the cholesterol efflux from the cells. The mechanisms of cholesterol transfer between cell membranes and underlying reason of gradient of cholesterol concentration between intracellular and plasma membranes will be discussed. We also describe four known mechanisms of cholesterol effluxaqueous diffusion, facilitated diffusion mediated by SR-B1 receptor, and active unidirectional efflux mediated by ABCA1 and ABCG1 transporters. The contribution of different pools of cholesterol and types of acceptor will be also considered.
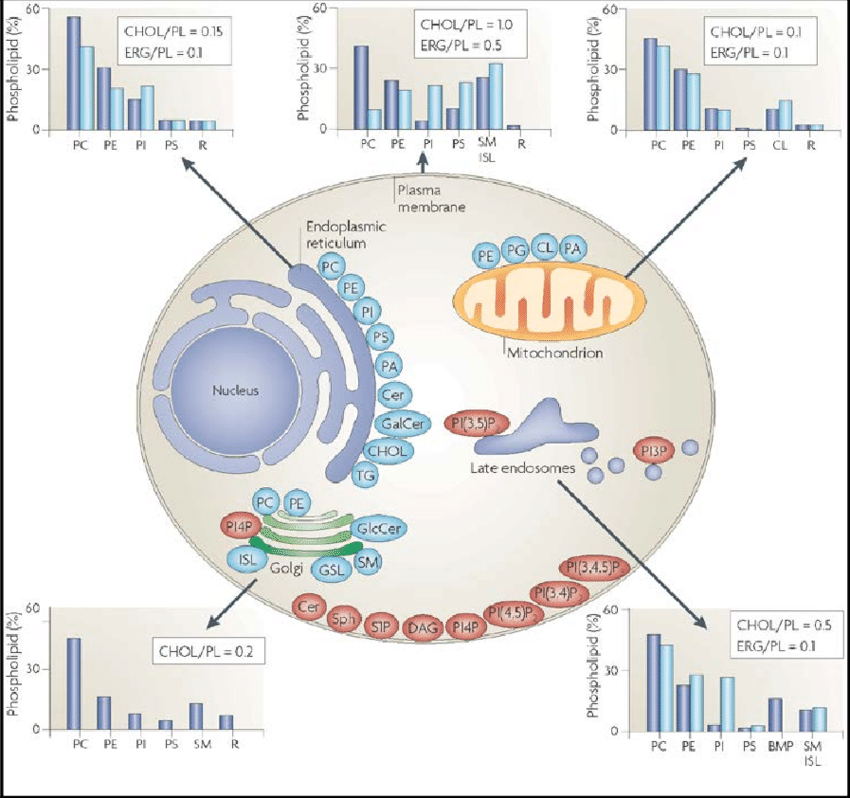
2. Lipid Rafts and Cholesterol Pools in Lipid Bilayer and Cell Membranes
2.1. Membrane Lipid Composition and Two Kinetic Pools of PM Cholesterol
2.2. Cholesterol Homeostasis Might Be Regulated by Active Cholesterol
3. Intracellular Cholesterol Turnover
3.1. Abundance of Cholesterol Pools
3.2. Vesicular and Nonvesicular Cholesterol Traffic
3.3. Lipid Transfer Proteins
3.4. Caveolae Cholesterol Is Actively Consumed in Cholesterol Efflux from the Cells
4. Molecular Mechanisms of Cholesterol Efflux
| Cells | Contribution of the pathway to efflux, % | Reference |
regulating calmodulin function
4.1. Aqueous Diffusion
4.2. ABCA1
4.3. ABCG1
4.4. SR-B1
You May Like: Is Shellfish High In Cholesterol
Enzymes Involved In Sterol And Steroid Synthesis
Having found high levels in adrenal smooth microsomes of all translocon subunits and associated elements examined, except for ribosomal protein and TRAP, it seemed important to confirm that preferential localization in smooth microsomes of enzymes involved in sterol and steroid synthesis was retained in these subcellular fractions. Therefore, we examined the levels of HMGR and two enzymes involved in steroid synthesis, 3HSD and 17-hydroxylase, a cytochrome P450 . All of these enzymes were in highest concentration in the adrenal smooth microsomes . None were detectable in corun rough microsomes from liver or pancreas, although HMGR was present in liver smooth and intermediate microsomes .
Microsomal Subfraction Protein And Ribosome Content
As expected, based on the morphology of the cells , smooth microsomes comprised a considerably greater percentage of the total microsomal fraction prepared from adrenals of control animals than from the liver, whereas rough microsomes were more abundant in liver . However, when animals were treated with xenobiotics, which induce the SER in hepatocytes, the liver smooth microsomal fraction increased, reaching levels comparable with those in adrenal smooth microsomes.
The smooth microsomal subfraction represents over 75% of total microsomal proteins obtained from adrenal tissue, but 2535% of control liver total microsomes. Treatment of animals with agents known to increase CYPs and other enzymes involved in xenobiotic metabolism increased the relative amount of smooth microsomes from liver tissue 2-fold. The percent of total microsomal protein in each microsomal fraction was calculated for fractions obtained from control guinea pig adrenal and for liver tissue from control guinea pigs and rats. These were compared with corresponding data derived from liver tissue of guinea pigs treated with 3MC, known to induce CYP1A, and rats treated with PB, known to induce CYP2B. The values shown represent the mean ± sem of three microsomal preparations for guinea pig liver and four for guinea pig adrenal and rat liver.
Recommended Reading: Bananas Lower Cholesterol
Sterols: 1 Cholesterol And Cholesterol Esters
In animal tissues, cholesterol is by far the most abundant member of a family of polycyclic compounds known as sterols. It can also be described as a polyisoprenoid or a triterpene from its biosynthetic origin. Cholesterol was first recognized as a component of gallstones as long ago as 1769, while the great French lipid chemist Chevreul isolated it from animal fats in 1815. However, it was well into the 20th century before the structure was fully defined by the German Chemist Heinrich Wieland, who received the Nobel Prize in Chemistry for his work in 1927, the first of thirteen so honoured for research on cholesterol and its metabolism.
Cholesterol plays a vital role in animal life, and it is essential for the normal functioning of cells both as a structural component of cell membranes and as a precursor of steroid hormones and other key metabolites including vitamin D and bile acids.It is also important for cell signalling, transport processes, nerve conduction and the regulation of gene transcription.Every cell in vertebrates is able both to synthesise cholesterol and to metabolize it, and there is evidence that synthesis de novois essential whatever the dietary intake this is vital in the brain.However, excess cholesterol can contribute to the pathology of various diseases, notably cardiovascular disease, so cholesterol levels must be balanced to ensure an adequate but not excessive supply.
Cellular Regulation Of Cholesterol
Cells tightly control the amount of cholesterol they synthesize and take up from the external environment. The expression of genes that encode proteins involved in the synthesis of cholesterol and uptake of cholesterol is regulated by the sterol response element binding protein . SREBP binds to sequences upstream of these genes, called sterol response elements , and activates transcription. Cholesterol regulates the activity of SREBP.
When initially synthesized, SREBP is a membrane protein that resides in the ER. It contains two transmembrane domains and two DNA-binding domains facing the cytosol. When cholesterol levels are high, SREBP is held in the ER through interactions with other proteins.
The proteins that keep SREBP in the ER contain a sterol-sensing domain. This domain binds cholesterol and when associated with cholesterol, assumes a conformation that allows other parts of the protein to interact with SREBP. When cholesterol levels fall, the sterol-sensing domain loses its interaction with cholesterol and changes conformation. This change in conformation causes the protein to dissociate from SREBP.
You May Like: How Much Cholesterol In Pork Chops